Estimate the vapor pressure of acetone (mm Hg) at 50C (a) from data in Perrys Chemical Engineers
Question:
Estimate the vapor pressure of acetone (mm Hg) at 50°C (a) from data in Perry’s Chemical Engineers’ Handbook (Footnote 1) and the Clausius–Clapeyron equation, (b) from the Antoine equation using parameters from Table B.4, and (c) using the AntoineP function in APEx.
Table B.4
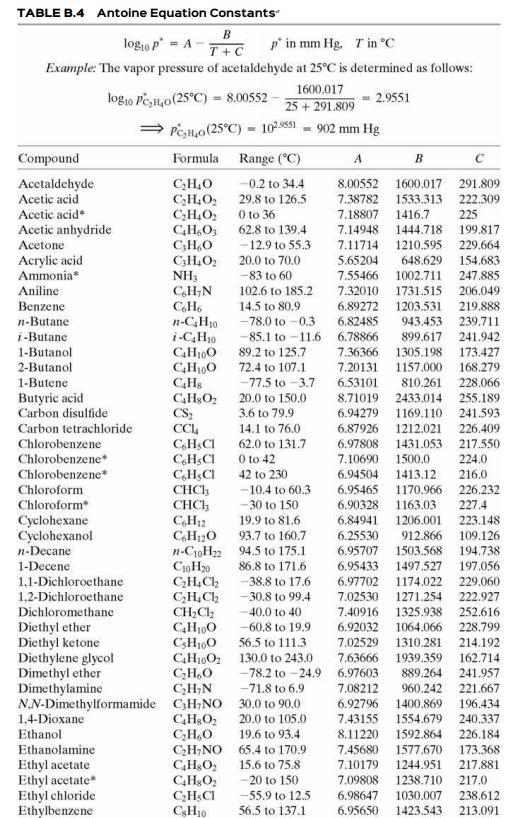
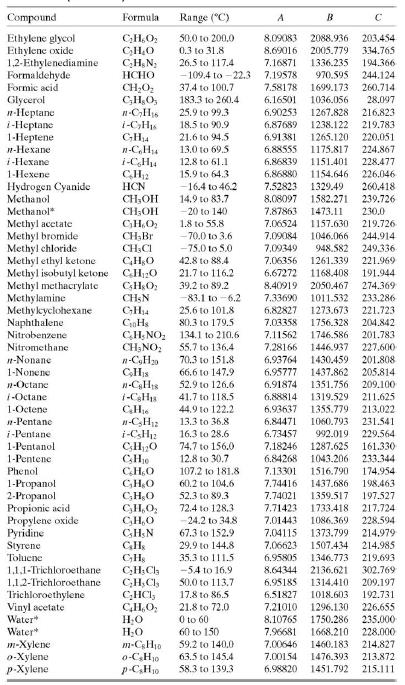
TABLE B.4 Antoine Equation Constants" B log,o p = A p' in mm Hg. Tin °C T+C Example: The vapor pressure of acetaldehyde at 25°C is determined as follows: 1600.017 log10 Pe,H,o(25°C) = 8.00552 25 + 291.809 2.9551 Pen,o(25°C) = 10s51 - 902 mm Hg Compound Formula Range (°C) A B Acctaldehyde Acetic acid C,H,0 C;H,O, C,H,O2 CH,O, C;H,O C3H,O2 NH3 C,H;N -0.2 to 34.4 8.00552 1600.017 291.809 29.8 to 126.5 O to 36 62.8 to 139.4 7.38782 1533.313 222.309 Acetic acid* 7.18807 1416.7 225 Acetic anhydride Acetone 7.14948 1444.718 1210.595 648.629 1002.711 199.817 - 12.9 to 55.3 7.11714 229,664 Acrylic acid Ammonia* 20.0 to 70.0 5.65204 154.683 -83 to 60 7.55466 247.885 Aniline 102.6 to 185.2 7.32010 1731.515 206.049 Benzene 14.5 to 80.9 -78.0 to -0.3 6.89272 1203.531 219.888 n-Butane 6.82485 943.453 n-C,H30 i-C,H10 239.711 i-Butane -85.1 to -11.6 6.78866 89.2 to 125.7 72.4 to 107.1 -77.5 to -3.7 899.617 241.942 1-Butanol 2-Butanol 1-Butene 7.36366 1305.198 173.427 168.279 CH0 CHs 7.20131 1157.000 6.53101 810.261 8.71019 2433.014 1169.110 1212.021 228.066 Butyric acid Carbon disulfide 20.0 to 150.0 3.6 to 79.9 255.189 241.593 CS; CC, C,HSCI C,H;CI C,H;CI CHCH CHCI; C,H12 C,H120 n-C10H2 94.5 to 175.1 CioH20 C;H,Ch 6.94279 Carbon tetrachloride 14.1 to 76.0 6.87926 226.409 Chlorobenzene 62.0 to 131.7 6.97808 1431.053 217.550 Chlorobenzene* 0 to 42 7.10690 1500.0 224.0 Chlorobenzene* 42 to 230 6.94504 1413.12 216.0 Chloroform -10.4 to 60.3 6.95465 1170.966 226.232 Chloroform -30 to 150 6.90328 1163.03 227.4 223.148 Cyclohexane Cyclohexanol 19.9 to 81.6 6.84941 1206.001 93.7 to 160.7 6.25530 912.866 109.126 n-Decane 194.738 6.95707 6.95433 1497.527 1503.568 1-Decene 86.8 to 171.6 197.056 1,1-Dichloroethane 1,2-Dichloroethane Dichloromethane - 38.8 to 17.6 6.97702 1174.022 229.060 -30.8 to 99.4 7.02530 1271.254 222.927 CH;Cl2 C,H100 C;H100 GH1002 130.0 to 243.0 CH,O C;H,N 40.0 to 40 7.40916 1325.938 1064.066 1310.281 252.616 228.799 Diethyl ether Diethyl ketone Diethylene glycol Dimethyl ether Dimethylamine N.N-Dimethylformamide C;H;NO 30.0 to 90.0 -60.8 to 19.9 56.5 to 111.3 6.92032 7.02529 214.192 -78.2 to -24.9 6.97603 -71.8 to 6.9 7.63666 1939.359 889.264 960.242 162.714 241.957 7.08212 221.667 6.92796 1400.869 7.43155 1554.679 196.434 1,4-Dioxane Ethanol C,H&O; CH,O C;H;NO 65.4 to 170.9 20.0 to 105.0 240.337 19.6 to 93.4 8.11220 1592.864 226.184 Ethanolamine 7.45680 1577.670 173.368 Ethyl acetate Ethyl acetate Ethyl chloride Ethylbenzene 15.6 to 75.8 7.10179 1244.951 217.881 -20 to 150 7.09808 1238.710 217.0 C;H;CI CH10 -55.9 to 12.5 6.98647 1030.007 238.612 56.5 to 137.1 6.95650 1423.543 213.091
Step by Step Answer:
Estimation of vapor pressure Vapor pressure is a measure of tendency of material change into the gas...View the full answer



Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Sciences questions
-
At 300C, the vapor pressure of Hg is 32.97 torr. What mass of Au would have to be dissolved in 5.00 g of Hg to lower its vapor pressure to 25.00 torr?
-
At 300C, the vapor pressure of Hg is 32.97 torr. If 0.775 g of Au were dissolved into 3.77 g of Hg, what would be the vapor pressure of the solution?
-
The Dew Point The vapor pressure of water (see Problem 18.88) decreases as the temperature decreases. If the amount of water vapor in the air is kept constant as the air is cooled, a temperature is...
-
Find the equation for the lower half of the circle x + (y-8) = 7. Put the equation in the form y = g(x), and then enter g(x) into the answer box below. Enter your answer as a symbolic function of x,...
-
Refer to Figure 12.8, which indicates ÎG for each glycolytic reaction under intracellular conditions. Assume that glyceraldehyde-3-phosphate dehy-drogenase was inhibited with iodoacetate, which...
-
A/an ________ rsum highlights employment experience, listing jobs in reverse order from most recent to earliest.
-
Use appropriate data from Appendix E to calculate the radiation pressure on a light-absorbing object at the Suns surface.
-
Using OLAP Query 1 shown in Figure 9.11 (based on data from Figure 9.7) as a starting point, give an example that illustrates a drill down operation.
-
Zach attended Biola University during 2015-2019. He lived at home and was claimed by his parents as a dependent during his entire education. He incurred education expenses of $20,000 during college,...
-
The Firm Chair Company manufactures customized wood furniture and sells the furniture in large quantities to major furniture retailers. Jim Bolling has recently been assigned to analyze the companys...
-
The vapor pressure of ethylene glycol at several temperatures is given below: (a) Construct a semilog plot of the vapor-pressure data and determine a linear expression for ln p* as a function of...
-
Estimate the vapor pressure of acetone (mm Hg) at 50C (a) from data in Perrys Chemical Engineers Handbook (Footnote 1) and the ClausiusClapeyron equation, (b) from the Antoine equation using...
-
From the Internet find examples of distribution centers or warehouses and list the functions that they serve?
-
1.Using the Excel file Sales transaction find the following15 Marks a.Identify the levels of measurement for each variables b.Construct a cross tabulation to find the number of transactions by...
-
Jason is a sole trader in the architecture industry. For the year ending 30 June 2019, Jason hired a 3D model designer, Sarah, to help him with the growing business. At the end of the year he has the...
-
Read Case 14-1 Trojan Technologies (15th ed., p. 426 OR 16th ed., p. 431) Guiding Questions and additional information: In preparing your case study, ensure that you answer the following questions:...
-
Jorge Rimert works for Road to Success Collection Agency. He oversees mailing out collection notices to patients. Upon review of the patients who have not paid from Hideaway Hospital, Jorge notices...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 99% confident that the estimated percentage is in error...
-
Write a recursive method that returns the value of N! (N factorial) using the definition given in this chapter. Explain why you would not normally use recursion to solve this problem.
-
Outline a general process applicable to most control situations. Using this, explain how you would develop a system to control home delivery staff at a local pizza shop.
-
A mixture of sulfuric acid and nitric acid will produce small quantities of the nitronium ion (NO2 + ): Does the nitronium ion have any significant resonance structures? Why or why not? o==o:
-
Consider the structure of ozone: Ozone is formed in the upper atmosphere, where it absorbs short-wavelength UV radiation emitted by the sun, thereby protecting us from harmful radiation. Draw all...
-
Identify the reagents you would use to achieve each of the following transformations: a) Convert tert-butyl bromide into a primary alkyl halide b) Convert 2-bromopropane into 1-bromopropane
-
AR by Company To see companies within a particular country, elther click on that country or use the search field in the upper left corner. To return to the map's global view, click on the Home icon...
-
Comprehensive Project for WACC and NPV Company A is considering a 4 - year project. They own a piece of land that could be sold for $ 1 0 0 , 0 0 0 . They hired an engineer to evaluate it and she has...
-
can anyone help find a news article discussing a corporations use of debt financing? Often times the financial news media will report when a company chooses to issue new bonds or take new loans.

Study smarter with the SolutionInn App


