The vapor pressure of ethylene glycol at several temperatures is given below: (a) Construct a semilog plot
Question:
The vapor pressure of ethylene glycol at several temperatures is given below:
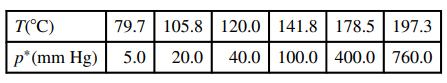
(a) Construct a semilog plot of the vapor-pressure data and determine a linear expression for ln p* as a function of 1/T(K). Use the results to estimate the heat of vaporization (kJ/mol) of ethylene glycol, and then use that value in the Clausius–Clapeyron equation to estimate the vapor pressures at each of the temperatures given in the table.
(b) Repeat Part (a) using the Slope and Intercept functions of APEx to obtain the expression for ln p* vs. 1=T(K).
(c) Use the results from Part (b) to estimate vapor pressures of ethylene glycol at 50°C, 80°C, and 110°C. Also estimate the boiling point of this substance at system pressures of 760 mm Hg and 2000 mm Hg. Compare all five results with those obtained directly using APEx functions. In which of the estimates at the given temperatures and pressures would you have the least confidence? Explain your reasoning.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





