Question: Ever wonder why espresso costs much more per cup than regular drip coffee? Part of the reason is the expensive equipment needed to brew a
Ever wonder why espresso costs much more per cup than regular drip coffee? Part of the reason is the expensive equipment needed to brew a proper espresso. A high-powered burr grinder first shears the coffee beans to a fine powder without producing too much heat. (Heating the coffee in the grinding stage prematurely releases the volatile oils that give espresso its rich flavor and aroma.) The ground coffee is put into a cylindrical container called a gruppa and tamped down firmly to provide an even flow of water through it. An electrically heated boiler inside the espresso machine maintains water in a reservoir at 1.4 bar and 109°C. An electric pump takes cold water at 15°C and 1 bar, raises its pressure to slightly above 9 bar, and feeds it into a heating coil that passes through the reservoir. Heat transferred from the reservoir through the coil wall raises the water temperature to 96°C. The heated water flows into the top of the gruppa at 96°C and 9 bar, passes slowly through the tightly packed ground beans, and dissolves the oils and some of the solids in the beans to become espresso, which decompresses to 1 atm as it exits the machine. The water temperature and uniform flow through the bed of packed coffee in the gruppa lead to the more intense flavor of espresso relative to normal drip coffee. Water drawn directly from the reservoir is expanded to atmospheric pressure where it forms steam, which is used to heat and froth milk for lattes and cappuccinos.
(a) Sketch this process, using blocks to represent the pump, reservoir, and gruppa. Label all heat and work flows in the process, including electrical energy.
(b) To make a 14-oz latte, you would steam 12 ounces of cold milk (3°C) until it reaches 71°C and pour it over 2 ounces of espresso. Assume that the steam cools but none of it condenses as it bubbles through the milk. For each latte made, the heating element that maintains the reservoir temperature must supply enough energy to heat the espresso water plus enough to heat the milk, plus additional energy. Assuming
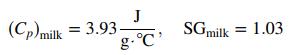
calculate the quantity of electrical energy that must be provided to the heating element to accomplish those two functions. Why would more energy than what you calculate be required?
(c) Coffee beans contain a considerable amount of trapped carbon dioxide, not all of which is released when the beans are ground. When the hot pressurized water percolates through the ground beans, some of the carbon dioxide is absorbed in the liquid. When the liquid is then dispensed at atmospheric pressure, fine CO2 bubbles come out of solution. In addition, one of the chemical compounds formed when the coffee beans are roasted and extracted into the espresso is melanoidin, a surfactant. Surfactant molecules are asymmetrical, with one end being hydrophilic (drawn to water) and the other end hydrophobic (repelled by water). When the bubbles (thin water films containing CO2) pass through the espresso liquid, the hydrophilic ends of the melanoidin molecules attach to the bubbles and the dissolved bean oils in turn attach to the hydrophobic ends. The result is that the bubbles emerge coated with the oils to form the crema, the familiar reddishbrown stable foam at the surface of good espresso. Speculate on why you don’t see crema in normal drip coffee. (Henry’s law should show up in your explanation.) Note: All soaps and shampoos contain at least one surfactant species. (A common one is sodium lauryl sulfate.) Its presence explains why if you have greasy hands, washing with plain water may leave the grease untouched but washing with soap removes the grease.
(d) Explain in your own words (i) how espresso is made, (ii) why espresso has a more intense flavor than regular drip coffee, (iii) what the crema in espresso is, how it forms, and why it doesn’t appear in regular drip coffee, and (iv) why washing with plain water does not remove grease but washing with soap does. (Many people automatically assume that all chemical engineers are extraordinarily intelligent. If you can explain those four things, you can help perpetuate that belief.)
(Cp)milk = 3.93- g- C SGmilk = 1.03
Step by Step Solution
3.37 Rating (163 Votes )
There are 3 Steps involved in it
a A sketch of the espressomaking process could include the following blocks a burr grinder a cylindrical gruppa an electrically heated reservoir an el... View full answer

Get step-by-step solutions from verified subject matter experts


