Molecules such as dimethylsulfoxide and dimethylsulfone can either be represented as hypervalent, that is, with more than
Question:
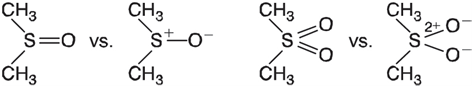
Atomic charges obtained from quantum chemical calculations can help to decide which representation is more appropriate.
a. Obtain equilibrium geometries for dimethylsulfide, (CH3)2S, and dimethylsufoxide using the HF/3-21G model and obtain charges at sulfur based on fits to the electrostatic potential. Is the charge on sulfur in dimethylsulfoxide about the same as that on sulfur in dimethylsulfide (normal sulfur), or has it increased by one unit, or is it somewhere between? Would you conclude that dimethylsulfoxide is best represented as a hypervalent molecule, as a zwitterion, or something between? See if you can support your conclusion with other evidence (geometries, dipole moments, and so on).
b. Repeat your analysis for dimethylsulfone. Compare your results for the charge at sulfur to those for dimethylsulfide and dimethylsulfoxide.
Step by Step Answer:






