Polyvinylpyrrolidone (PVP) is a polymer product used as a binding agent in pharmaceutical applications as well as
Question:
Polyvinylpyrrolidone (PVP) is a polymer product used as a binding agent in pharmaceutical applications as well as in personal-care items such as hairspray. In the manufacture of PVP, a spray-drying process is used to collect solid PVP from an aqueous suspension, as shown in the flowchart on the next page. A liquid solution containing 65 wt% PVP and the balance water at 25°C is pumped through an atomizing nozzle at a rate of 1500 kg/h into a stream of preheated air flowing at a rate of 1.57 × 104 SCMH.
The water evaporates into the stream of hot air and the solid PVP particles are suspended in the humidified air. Downstream, the particles are separated from the air with a filter and collected. The process is designed so that the exiting solid product and humid air are in thermal equilibrium with each other at 110°C. For convenience, the spray-drying and solid-separation processes are shown as one unit that may be considered adiabatic.
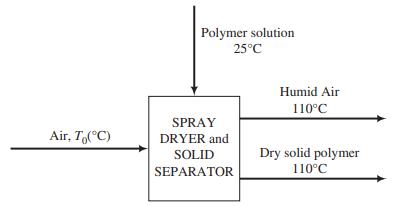
(a) Draw and completely label the process flow diagram and perform a degree-of-freedom analysis.
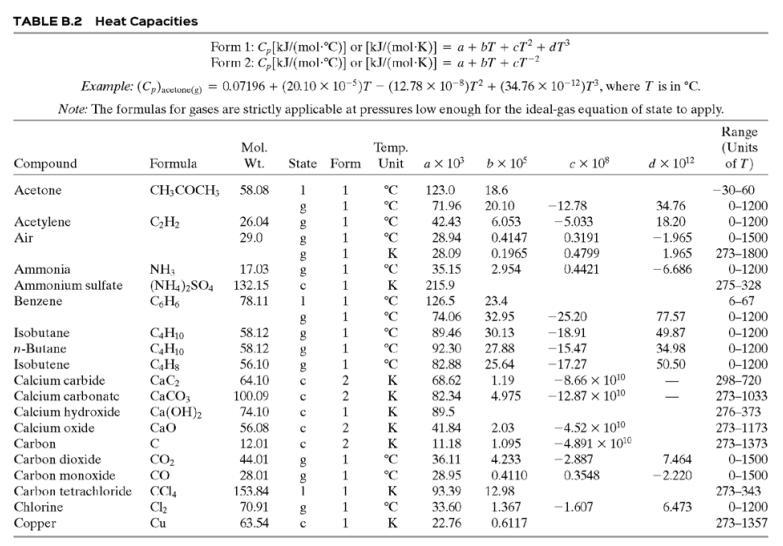
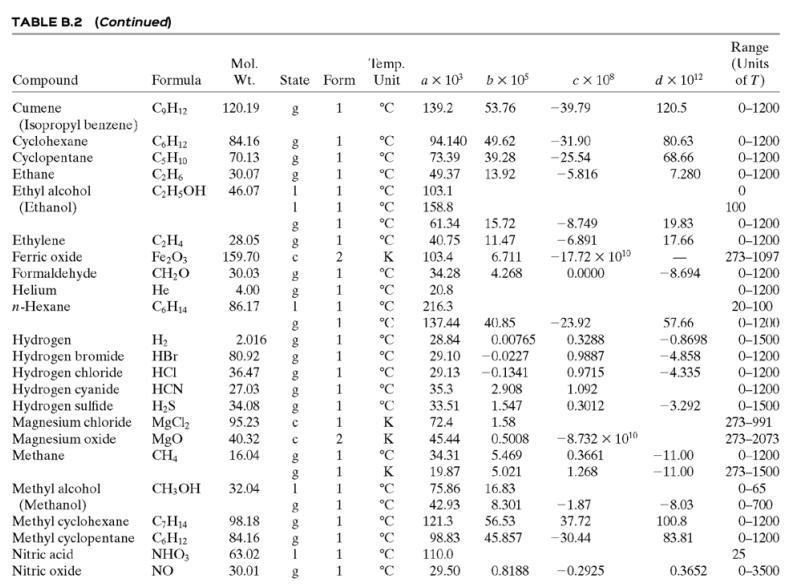
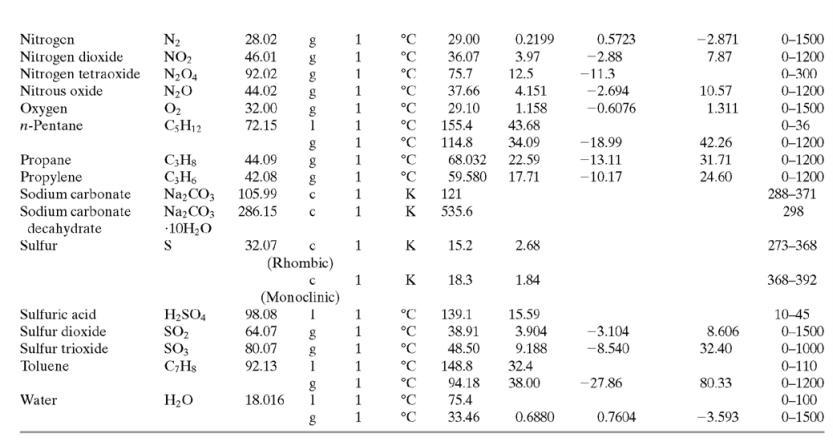
(b) Calculate the required temperature of the inlet air, T0, and the volumetric flow rate (m3/h) and relative humidity of the exiting air. Assume that the polymer has a heat capacity per unit mass onethird that of liquid water, and only use the first two terms of the polynomial heat-capacity formula for air in Table B.2.
(c) Why do you think the polymer solution is put through an atomizing nozzle, which converts it to a mist of tiny droplets, rather than being sprayed through a much less costly nozzle of the type commonly found in showers?
(d) Due to a design flaw, the polymer solution does not remain in the dryer long enough for all the water to evaporate, so the solid product emerging from the separator is a wet powder. How will this change the values of the outlet temperatures of the emerging gas and powder and the volumetric flow rate and relative humidity of the emerging gas (increase, decrease, can’t tell without doing the calculations)? Explain your answers.
Table B.2
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard




