Question: Sodium hydroxide is dissolved in enough water to make up a 20.0 mole% solution. (a) If the NaOH and water are initially at 77F (25C),
Sodium hydroxide is dissolved in enough water to make up a 20.0 mole% solution.
(a) If the NaOH and water are initially at 77°F (25°C), how much heat (Btu/lb product solution) must be removed for the solution also to be at 77°F. Assume the process is carried out at constant pressure, so that Q = ΔH, and use Table B.11 to evaluate ΔĤs.
(b) If the dissolution is done adiabatically, estimate the final temperature of the solution. Assume that the heat capacity of the solution is approximately that of pure liquid water.
(c) If the process of Part (b) were actually carried out, the final temperature would be less than the value calculated. Why? (Neglect errors caused by the assumptions of adiabatic dissolution and a solution heat capacity equal to that of pure water.)
Table B.11
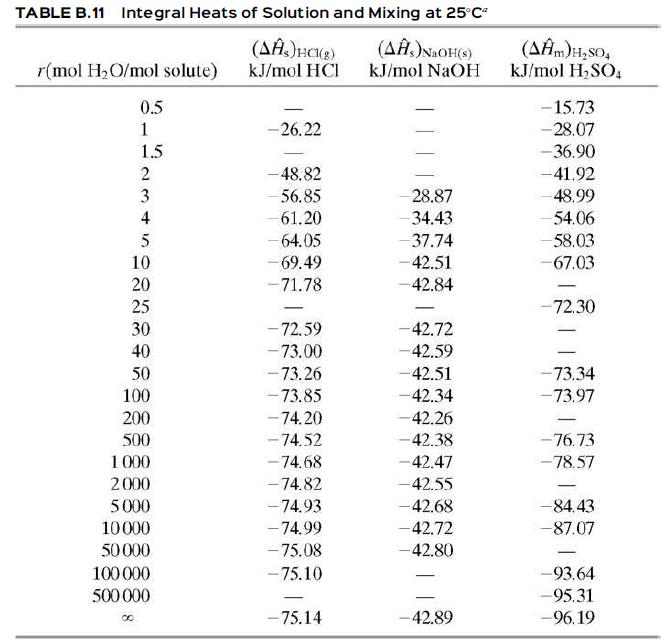
TABLE B.11 Integral Heats of Solution and Mixing at 25C" (A)NaOH(s) kJ/mol NaOH r(mol H,O/mol solute) kJ/mol HCI kJ/mol H,SO, 0.5 -15.73 1 -26.22 -28.07 1.5 -36.90 2 -48.82 -41.92 56.85 28.87 48.99 4 61.20 -34.43 54.06 5 - 64.05 -37.74 58.03 10 -69.49 -42.51 -67.03 20 - 71.78 -42.84 25 -72.30 - - 72.59 - 73.00 - 73.26 30 -42.72 40 -42.59 50 100 -42.51 -73.34 - 73.85 - 74.20 - 74.52 -42.34 -73.97 200 -42.26 500 -42.38 -76.73 1 000 -74.68 -42.47 -78.57 2000 - 74.82 -42.55 - 5000 -74.93 -42.68 -84.43 - 74.99 - 75.08 - 75.10 10000 -42.72 -87.07 50000 -42.80 100 000 -93.64 500 000 -95.31 -75.14 -42.89 -96.19
Step by Step Solution
3.49 Rating (162 Votes )
There are 3 Steps involved in it
a Heat must be removed from the solution to maintain a constant temperature of 77F 25C ... View full answer

Get step-by-step solutions from verified subject matter experts


