The bond angle about oxygen in alcohols and ethers is typically quite close to tetrahedral (109.5°), but
Question:
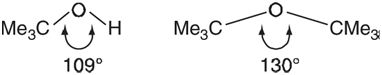
This is entirely consistent with the notion that while lone pairs take up space, they can be €œsqueezed€ to relieve crowding. Another way to relieve unfavorable steric interactions (without changing the position of the lone pairs) is to increase the C~O bond distance.
a. Build tert-butyl alcohol and di-tert-butyl ether and optimize the geometry of each using the HF/6-31G* model. Are the calculated bond angles involving oxygen in accord with the values given earlier, in particular with regard to the observed increase in bond angle? Do you see any lengthening of the C€•O bond in the ether over that in the alcohol? If not, or if the effect is very small (<0.01 Ã…), speculate why not.
b. Next, consider the analogous trimethylsilyl compounds Me3SiOH and Me3SiOSiMe3. Calculate their equilibrium geometries using the HF/6-31G* model. Point out any similarities and any differences between the calculated structures of these compounds and their tert-butyl analogues. In particular, do you see any widening of the bond angle involving oxygen in response to increased steric crowding? Do you see lengthening of the SiۥO bond in the ether over that of the alcohol? If not, rationalize what you do see.
Step by Step Answer:






