Water contains two acidic hydrogens that can act as hydrogen-bond donors and two lone pairs that can
Question:
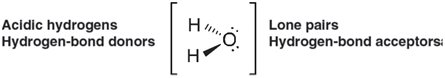
Given that all are tetrahedrally disposed around oxygen, this suggests two reasonable structures for the hydrogen-bonded dimer of water, (H2O)2, one with a single hydrogen bond and one with two hydrogen bonds:
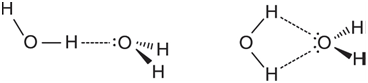
Whereas the second seems to make better use of water€™s attributes, in doing so it imposes geometrical restrictions on the dimer. Build the two dimer structures. Take into account that the hydrogen-bond distance (O. . . H) is typically on the order of 2 Ã…. Optimize the geometry of each using the HF/6-31G* model and, following this, calculate vibrational frequencies.
Which structure, singly or doubly hydrogen bonded, is more stable? Is the other (higher-energy) structure also an energy minimum? Explain how you reached your conclusion. If the dimer with the single hydrogen bond is more stable, speculate what this has told you about the geometric requirements of hydrogen bonds. Based on your experience with water dimer, suggest a €œstructure€ for liquid water.
Step by Step Answer:






