What do you expect the electronic spectrum to look like for the ground and excited states shown
Question:
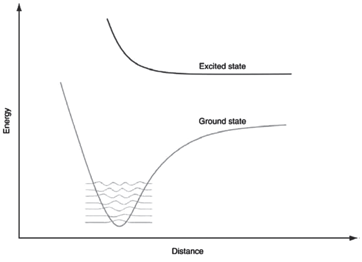
Transcribed Image Text:
Excited state Ground state Distance ABsoug
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
The electronic spectrum will have no ...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Do you expect the viscosity of glycerol, C3H5(OH)3, to be larger or smaller than that of 1-propanol, C3H7OH? Explain? (a) Glycerol (b) 1-Propanol
-
Consider the tetrahedral anions VO43- (orthovanadate ion), GrO42- (chromate ion), and MnO4 (permanganate ion). (a) These anions are isoelectronic. What does this statement mean? (b)Would you expect...
-
Do you expect the light given off by (a) a neon sign or (b) an incandescent lightbulb to be continuous in distribution or in the form of a line spectrum? Explain.
-
A manufacturing company reports the following information for the month of May. Note: Assume all raw materials were used as direct materials. Activities for May Advertising expense Raw materials...
-
In Exercises 1-4, find the exact values of the six trigonometric functions of the angle . 1. 2. 3. 4. 8. 13
-
The line l with gradient 3 and y-intercept (0, 5) has the equation ax 2y + c = 0. Find the values of a and c.
-
What was the precursor to globalization, and why was it important? L01
-
Using the data presented in BE13-4 for Rosalez Company, perform vertical analysis.
-
Question 2: Hedge Accounting You have been recently hired as a staff accountant at Global Design, Inc., a small chain of retail home furnishing stores. You report directly to the Chief Financial...
-
An output interface in a switch is designed using the leaky bucket algorithm to send 8000 bytes/s (tick). If the following frames are received in sequence, show the frames that are sent during each...
-
Because internal conversion is in general very fast, the absorption and fluorescence spectra are shifted in frequency as shown in Figure 25.10. This shift is crucial in making fluorescence...
-
Why are the spectra of the individual molecules in the bottom trace of Figure 25.15 shifted in frequency? 10,000 Molecules x1 1,000 x10 x100 100 10 Frequency Absorption
-
Blood contains three types of cells: red blood cells, white blood cells, and platelets. For approximately every 600 red blood cells in healthy humans, there are 40 platelets and 1 white blood cell....
-
Jimmy Joe-Bob Hicky is the district commander for the mostly-rural Spud Valley highway patrol district in western Idaho. Hes attempting to assign highway patrol cars to different road segments in his...
-
Its important to have a holistic view of all the businesses combined and ensure that the desired levels of risk management and return generation are being pursued. Agree or disagree
-
(3pts each) During a trip to a casino, Adam Horovitz plays his favorite casino game 10 times. Each time he plays, he has a 41% chance of winning. Assume plays of the game are independent. a. What is...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 95% confident that the estimated percentage is in error...
-
Statement of financial position as at 31 December 2014 ASSETS Non-current assets Property, plant and equipment Delivery van at cost 12,000 Depreciation (2,500) 9,500 Current assets Inventories...
-
In Exercises 1 through 28, differentiate the given function. 1 8 f(x) = 11/16 4 2 - x + 2
-
U.S. households have become smaller over the years. The following table from the 2010 GSS contains information on the number of people currently aged 18 years or older living in a respondent's...
-
For how long on average would an H atom remain on a surface at 298 K if its desorption activation energy were (a) 15 kJ mol 1 , (b) 150 kJ mol 1 ? Take 0 = 0.10 ps. For how long on average would the...
-
The adsorption of a gas is described by the Langmuir isotherm with K = 0.85 kPa 1 at 25C. Calculate the pressure at which the fractional surface coverage is (a) 0.15, (b) 0.95.
-
(a) Discuss the main structural features of the electrical double layer. (b) Distinguish between the electrical double layer and the Nernst diffusion layer.
-
firm purchased a new piece of equipment with an estimated useful life of eight years. The cost of the equipment was $65,000. The salvage value was estimated to be $10,000 at the end of year 8. Using...
-
On an average day, a company writes checks totaling $1,500. These checks take 7 days to clear. The company receives checks totaling $1,800. These checks take 4 days to clear. The cost of debt is 9%....
-
Olds Company declares Chapter 7 bankruptcy. The following are the book values of the asset and liability accounts at that time. A bankruptcy expert estimates that administrative expense will total $...

Study smarter with the SolutionInn App


