Why are the spectra of the individual molecules in the bottom trace of Figure 25.15 shifted in
Question:
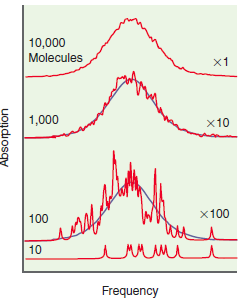
Transcribed Image Text:
10,000 Molecules x1 1,000 x10 x100 100 10 Frequency Absorption
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 93% (15 reviews)
In a solution different molecules do not all ...View the full answer

Answered By

Surojit Das
I have vast knowledge in the field of Mathematics, Business Management and Marketing. Besides, I have been teaching on the topics Management leadership, Business Administration, Human Resource Management, Business Communication, Accounting, Auditing, Organizer Behaviours, Business Writing, Essay Writing, Copy Writing, Blog Writing since 2020. It is my personality to act quickly in any emergency situations when students need my services. I am very professional and serious in every questions students asked me at the time of dealing any projects. I have been serving detailed, quality, properly analysed research paper through the years.
4.80+
91+ Reviews
279+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Does it make sense to distinguish individual NaCl molecules in a salt crystal? What about individual H 2 O molecules in an ice crystal? Explain.
-
The following spectra are taken from a compound that is an important starting material for organic synthesis. Determine the structure, first by considering each spectrum individually, then by...
-
Why is a type O individual considered a universal blood donor? Why is a type AB individual considered a universal acceptor?
-
For Questions consider the S-N curve provided below for this same material and situation. Stress & (MPa) 400 300 B. 175 MPa C. 200 MPa 200 100 10 C. 350 MPa D. 400 MPa E. It will never fail P-0.99...
-
In Exercises 1-4, use trigonometric identities to transform the left side of the equation into the right side (0 < < / 2). 1. tan cot = 1 2. cos sec = 1 3. tan cos = sin 4. cot sin = cos
-
The line joining (3, 2) to (2e, 5) has gradient 2. Work out the value of e.
-
What important changes happened during the second phase of globalization? L01
-
Ebanks, Brown, and Thomas are partners. They carry on a business jointly as EBT surveyors and share profits and losses in the ratio 25:45: 30. The trading account profit as at 31 December 2021 was...
-
Exerclse 1 8 - 1 9 ( Algo ) Stock dlvidend [ LO 1 8 - 8 ] The shareholders' equity of Core Technologies Company on June 3 0 , 2 0 2 3 , included the following:On April 1 , 2 0 2 4 , the board of...
-
Two wires P and Q both obey Hookes law. They are both stretched and have the same strain. The Young modulus of P is four times larger than that of Q. The diameter of P is twice that of Q. What is the...
-
What do you expect the electronic spectrum to look like for the ground and excited states shown in the figure below? Excited state Ground state Distance ABsoug
-
Suppose you obtain the UV photoelectron spectrum shown here for a gas-phase molecule. Each of the groups corresponds to a cation produced by ejecting an electron from a different MO. What can you...
-
The FOMC expects inflation to remain low in the near term, but to rise to 2 percent over the medium term. Where on the short-run Phillips curve does the Fed believe the economy to be? What is the Fed...
-
James A. and Ella R. Polk, ages 70 and 65, respectively, are retired physicians who live at 3319 Taylorcrest Street, Houston, Texas 77079. Their three adult children (Benjamin Polk, Michael Polk, and...
-
I need help solving the following question: - Thank you in advance. On January 1, Year 6, HD Lid., a building supply company, JC Lid., a construction company, and Mr. Saeid, a private investor,...
-
Let X 1 , , X n X 1 , , X n be a random sample from a normal distribution with mean and variance 1. Find the uniformly minimum variance unbiased estimator of 2 2 .2 answers
-
The ledger of Duggan Rental Agency on March 31 of the current year includes the following selected accounts before adjusting entries have been prepared. Debit Credit Prepaid Insurance $3,600 Supplies...
-
1.Using the Excel file Sales transaction find the following15 Marks a.Identify the levels of measurement for each variables b.Construct a cross tabulation to find the number of transactions by...
-
In Exercises 13 through 20, use the chain rule to compute the derivative dy/dx for the given value of x. y = u; u = x 2 2x + 6 r x = 3
-
Define a traverse in Surveying?
-
The average time for which an oxygen atom remains adsorbed to a tungsten surface is 0.36 s at 2548 K and 3.49 s at 2362 K. Find the activation energy for desorption. What is the pre-exponential...
-
The enthalpy of adsorption of CO on a surface is found to be 120 kJ mol 1 . Estimate the mean lifetime of a CO molecule on the surface at 400 K.
-
The following data have been obtained for the adsorption of H2 on the surface of 1.00 g of copper at 0C. The volume of H 2 below is the volume that the gas would occupy at STP (0C and 1 atm)....
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


