0.020 mol of gas undergoes the process shown in FIGURE EX18.37. a. What type of process is...
Question:
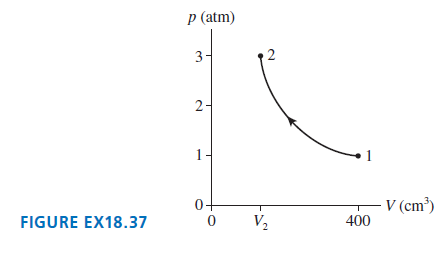 a. What type of process is this?
a. What type of process is this?
b. What is the final temperature in °C?
c. What is the final volume V2?
Transcribed Image Text:
p (atm) 3- 2- 1- V (cm³) 400 0+ V2 FIGURE EX18.37 2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (14 reviews)
Model Assume that the gas is an ideal gas Solve a The graph shows that th...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Physics for Scientists and Engineers A Strategic Approach with Modern Physics
ISBN: 978-0133942651
4th edition
Authors: Randall D. Knight
Question Posted:
Students also viewed these Physics questions
-
0.0040 mol of gas undergoes the process shown in FIGURE EX18.35. a. What type of process is this? b. What are the initial and final temperatures in °C? p (atm) 3- 2- V (cm) 300 0+ 100 200 FIGURE...
-
0.0050 mol of gas undergoes the process 1?? 2 ?? 3 shown in Figure P16.38.? What are (a) Temperature T 1 , (b) Pressure p 2 , (c) Volume V 3 ? p (atm) P2 - T = 2926 K 3 T3 = 2438 K 1 0+ V (cm) V3...
-
A gas undergoes the process shown in FIGURE Q18.10. By what factor does the temperature change? FIGURE Q18.10
-
Mr. Mo carries on business as a sole proprietor. The fiscal year end of the business is December 31. During 2020, its first year of operation, net business loss amounts to $72,000. In addition, the...
-
Before Lemon Corporation was taken private in a transaction engineered by its largest stockholder, some of Lemon's employees had unexercised options to purchase stock of Lemon. Under an employee...
-
All contest entries should be sent to the business manager or (I, me, myself). Our goal is to invite the CEO and (her, she) as keynote speakers for the conference. If you were (I, me), would you...
-
(Comprehensive Income) Roxanne Carter Corporation reported the following for 2004: net sales $1,200,000; cost of goods sold $750,000; selling and administrative expenses $320,000; and an unrealized...
-
D-List Calendar Co. specializes in manufacturing calendars that depict obscure comedians. The company uses a standard cost system to control its costs. During one month of operations, the direct...
-
Required information Skip to question [The following information applies to the questions displayed below.] Sweeten Company had no jobs in progress at the beginning of March and no beginning...
-
As the Purchase Manager of R. K. Engg. Company, Mumbai, you had sent an order for 15 scanners to National Systems Ltd., Delhi, but you received only 12 scanners on delivery. Write a letter to the G....
-
A gas with an initial temperature of 900°C undergoes the process shown in FIGURE EX18.36. a. What type of process is this? b. What is the final temperature in °C? c. How many moles of gas are...
-
iRobot designs and manufactures robots for consumer, commercial, and military use. For the fiscal year ended January 2, 2016, the company reported the following on its balance sheet and income...
-
Calculation of cost-plus selling price and an evaluation of pricing decisions A firm manufactures two products EXE and WYE in departments dedicated exclusively to them. There are also three service...
-
Convex Productions produces full-length motion pictures for distribution worldwide. Convex has just purchased the rights to a movie script entitled Native Sun, which it intends to develop as its next...
-
You are visiting the Engineering Office of Denton Hospital, as part of a consulting project. You notice some charts on one wall which look familiar to you: One of the employees notices you reading...
-
Richmond Clinic has obtained the following estimates for its costs of debt and equity at different capital structures: What is the firms optimal capital structure? (Hint: Calculate its corporate cost...
-
Suppose a sample yields estimates \(\widehat{\theta}_{1}=5, \widehat{\theta}_{2}=3\), se \(\left[\widehat{\theta}_{1} ight]=2\), and se \(\left[\widehat{\theta}_{2} ight]=1\) and the correlation...
-
Helium expands in a nozzle from \(0.8 \mathrm{MPa}, 500 \mathrm{~K}\), and negligible velocity to \(0.1 \mathrm{MPa}\). Calculate the throat and exit areas for a mass flow rate of \(0.34 \mathrm{~kg}...
-
Solve the given problems. For the segment in Fig. 2.91, find the segment height h in terms of r. Fig. 2.91. A = area r ''h
-
Read the Forecasting Supply Chain Demand Starbucks Corporation case in your text Operations and Supply Chain Management on pages 484-485, then address the four questions associated with the...
-
Make a very rough estimate of the thermal energy content of Earth, assuming that the core has radius 3480 km, temperature 4000 K, density 11000 kg/m 3 and heat capacity 800 J/K kg, and that the rest...
-
Check eq. (32.4) by estimating the radioactive heat production rate using data from Table 20.6 Take the fractional abundances of 235 U, 40 K to be 0.72%, 0.012%. Take the average energy release in 40...
-
Assume that a region of continental crust has a typical surface heat flux of 65 mW/m 2 , crustal density of 2750 kg/m 3 , and typical crustal abundances of radioactive nuclides (as given in Table...
-
A project will generate annual cash flows of $237,600 for each of the next three years, and a cash flow of $274,800 during the fourth year. The initial cost of the project is $749,600. What is the...
-
You want to invest annual amounts over the next 15 years. If your goal is to have $15,000 at the end of that time and if you can earn 8 percent on your invested funds, how much do you need to invest...
-
please explain thoroughly how to do in excel 1. Find the number of units to ship from each factory to each customer that minimizes total cost

Study smarter with the SolutionInn App


