Question: FIGURE EX40.4 shows the wave function of an electron in a rigid box. The electron energy is 12.0 eV. What is the energy, in eV,
FIGURE EX40.4 shows the wave function of an electron in a rigid box. The electron energy is 12.0 eV. What is the energy, in eV, of the next higher state?
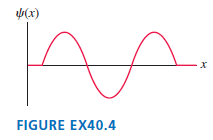
(x) FIGURE EX40.4
Step by Step Solution
3.36 Rating (152 Votes )
There are 3 Steps involved in it
Model Model the electron as a particle in a rigid onedi... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
1442_6054778c02a36_701146.pdf
180 KBs PDF File
1442_6054778c02a36_701146.docx
120 KBs Word File


