FIGURE P20.50 shows the thermal energy of 0.14 mol of gas as a function of temperature. What
Question:
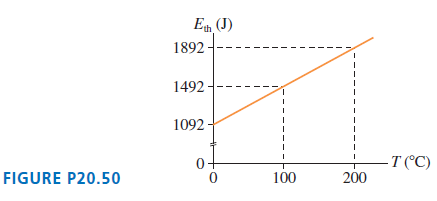
Transcribed Image Text:
E (J) 1892--- 1492 - 1092 T (°C) FIGURE P20.50 100 200
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (20 reviews)
Model Assume the gas is ideal so that Equation 2030 w...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Physics for Scientists and Engineers A Strategic Approach with Modern Physics
ISBN: 978-0133942651
4th edition
Authors: Randall D. Knight
Question Posted:
Students also viewed these Physics questions
-
The energy from nuclear fission appears in the form of thermal energy-but the thermal energy of what?
-
A cylinder of nitrogen gas has a volume of 15,000 cm 3 and a pressure of 100 atm. a. What is the thermal energy of this gas at room temperature (20C)? b. What is the mean free path in the gas? c. The...
-
A metal tool is sharpened by being held against the rim of a wheel on a grinding machine by a force of 180 N. The frictional forces between the rim and the tool grind off small pieces of the tool....
-
2) WWW.myitlab.com is an example of a(n). O domain name O protocol prefix OURL omni box
-
In the 1990s many Nasdaq firms favored growth over profitability; in the 2000s the goal of profitability is displacing growth. How might each preference be explained?
-
A company estimates that warranty expense will be 4% of sales. The company's sales for the current period are $246,000. The current period's entry to record the warranty expense is: Multiple Choice o...
-
how researchers used ANOVA to study the effects of pets at work
-
Abbe Co. is a small merchandising company with a manual accounting system. An investigation revealed that in spite of a sufficient bank balance, a significant amount of available cash discounts had...
-
- Prepare the journal entries to record these transactions: A. At the beginning of the year Mohammed, Inc. issued a number of shares of 5 par value stock for 30 per share. (Provide an amount of the...
-
Industrial Chemicals produces two adhesives used in the manufacturing process for airplanes. The two adhesives, which have different bonding strengths, require different amounts of production time:...
-
A 10 cm 10 cm 10 cm box contains 0.010 mol of nitrogen at 20C. What is the rate of collisions (collisions/s) on one wall of the box?
-
A 100 cm 3 box contains helium at a pressure of 2.0 atm and a temperature of 100C. It is placed in thermal contact with a 200 cm 3 box containing argon at a pressure of 4.0 atm and a temperature of...
-
In what respect may bankruptcy bring about the discharge of contracts?
-
Simon Company's year-end balance sheets follow. At December 31 Assets Cash Accounts receivable, net Merchandise inventory Prepaid expenses Plant assets, net Total assets Liabilities and Equity...
-
Openthe Phetsimulation Charges and Field from this link: ( https://phet.colorado.edu/en/simulation/charges-and-fields ) . In this simulation, a little different model is used: the little yellow "E...
-
A particle of mass m that is moving along the x-axis is experiencing a restoring force of the form F = -k+x, where kf is the spring constant. The Hamiltonian for this system is given as: = d 2m dx +...
-
Distinguish carefully between the following terms: i) Resolution and Sensitivity. ii) Type A and Type B evaluations of measurement uncertainty. [30%] (b) Describe two common schemes used for the...
-
Write out the form of the partial fraction decomposition of the function (See Example). Do not determine the numerical values of the coefficients. (a) +3 x5 + 2x3 A B C x Dx + E ++++ 2+2 6 (b)...
-
In Exercises find the interval of convergence of the power series. (Be sure to include a check for convergence at the endpoints of the interval.) o (5x)" n=0
-
For the following arrangements, discuss whether they are 'in substance' lease transactions, and thus fall under the ambit of IAS 17.
-
Is chemistry the study of submicroscopic, microscopic, or macroscopic matter, or of all three? Defend your answer.
-
You combine 50 mL of water with 50 mL of purified alcohol and get a total of 98 mL of the mixture. Please explain how this occurs.
-
A cotton ball is dipped in alcohol and wiped across a tabletop. Explain what happens to the alcohol molecules deposited on the tabletop. Is this a physical or chemical change?
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Difference between Operating Leverage and Financial Leverage
-
bpmn diagram for misc purchases

Study smarter with the SolutionInn App


