The graph below best represents the relationship between concentration and temperature for which of the following substances?
Question:
The graph below best represents the relationship between concentration and temperature for which of the following substances?
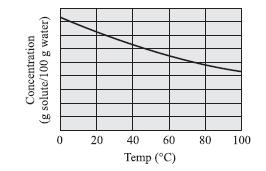
F. HCl
G. NaNO3
H. NaCl
J. KCl
Transcribed Image Text:
Concentration (g solute/100 g water) 0 20 40 60 Temp (°C) 80 100
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
The best answer is F In this question you are asked to look at the trends of the substances ...View the full answer

Answered By

Emel Khan
I have the ability to effectively communicate and demonstrate concepts to students. Through my practical application of the subject required, I am able to provide real-world examples and clarify complex ideas. This helps students to better understand and retain the information, leading to improved performance and confidence in their abilities. Additionally, my hands-on approach allows for interactive lessons and personalized instruction, catering to the individual needs and learning styles of each student.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Business questions
-
What are Parametric and Non- Parametric decision making techniques? Explain any two parametric and two non-parametric decision making methods.
-
You are an investment manager for Simple Asset Management, a company that specializes in developing simple investment portfolios consisting of no more than three assets such as stocks, bonds, etc.,...
-
Which graph below shows the expected relationship between temperature and flux (J, rate of flow) of an ion transported across a biological membrane via a carrier ionophore? Explain. femperature...
-
Prove the following statement: If a transformer having a series impedance Ze is connected as an autotransformer, its per-unit series impedance Z as an autotransformer will be SE Zoq NSE + NC Note...
-
Pattia Company purchased property with a warehouse and parking lot for $1,500,000. An appraiser valued the components of the property if purchased separately as follows. Land .............. $ 400,000...
-
In our text, the author discusses five drivers of a green supply chain. While each is important, different companies may be more influenced by some more than others. In your discussion, give an...
-
When valuing minority interests of private companies, should appraisers normalize earnings? a. What are normalizing adjustments? b. What kinds of normalizing adjustments should be made? c. What is...
-
Sally and Charles Heck received the following Form 1099-DIV in 2018: The Hecks also received the following dividends and interest in 2018 (Forms 1099-DIV not shown): Assuming the Hecks file a joint...
-
Proforma of Two Years and its expected Scenarios Assumptions 2021 2022 Sales Growth 83.94% -4.18% Interest Expense Growth 26.64% 17.49% Selling General and Administrative expense 70.14% -4.89% Net...
-
Which of the following statements from the passage is an acknowledgment by the author that the Michael Nyman Band enjoys limited popularity? F. English composer Michael Nyman has emerged as one of...
-
The author claims Michael Nyman used the music for Gattaca to impose a trance on the audience (lines 6162) because: A. It reverberates with layers of emotional string melodies. B. It features...
-
PJ Corporation pays $5,400,000 for an 80 percent interest in Sof Corporation on January 1, 2011, at which time the book value and fair value of Sof's net assets are as follows (in thousands):...
-
On March 1 , Kerr Corporation issued 1 0 , 0 0 0 preferred shares for $ 1 0 0 per share. On July 1 5 , it issued an additional 3 0 , 0 0 0 shares for $ 1 2 0 per share. Each share is convertible into...
-
Hello, I need to calculate the break-even point in part B using the following formula: investment amount / (CLV of gold - CLV of platinum) . However, since that results in a negative value, does that...
-
Nervousness and depression are examples of __________ symptoms. psychophysiologic social health environmental psychological Roberta is using the structured format to present the results of his study...
-
Identify the stage of change the client is in and write in behavioral language at least one problem, with at least one goal and a minimum of two objectives for each goal for the client vignettes...
-
Photon Technologies, Inc., a manufacturer of batteries for mobile phones, signed a contract with a large electronics manufacturer to produce three models of lithium-ion battery packs for a new line...
-
Chene Inc. has just developed a new drill. The new product is expected to produce annual revenues of $1,350,000. To produce the drill requires an investment in new equipment costing $1,440,000 and...
-
In Exercises discuss the continuity of each function. f(x) -3 1 x - 4 y 3 2 -1 -2 -3+ 3 X
-
Betty incurs the following transactions during the current year. Without considering the transactions, her 2016 AGI is $40,000. Analyze the transactions and answer the following questions: On March...
-
Joe is a single, self-employed individual who owns his own business. During 2016 Joe reported $200,000 gross income and $60,000 expenses from his business. He also paid $30,000 in alimony to his...
-
For 2016, Mario, a single individual with no dependents, receives income of $55,000 and incurs deductible expenses of $9,000. a. What is Marios taxable income assuming that the expenses are...
-
Saly paid $52,000 a year paid on a weekly basis. last pay she had $250 withheld in Income Tax, $48.97 for CPP and $15.80 for EI. an additional $and 25.00 in tax are deducted each pay. She allowed to...
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...

Study smarter with the SolutionInn App


