A solid mixture weighing 0.05485 g contained only ferrous ammonium sulfate and ferrous chloride. The sample was
Question:
A solid mixture weighing 0.05485 g contained only ferrous ammonium sulfate and ferrous chloride. The sample was dissolved in 1 M H2SO4, and the Fe2+ required 13.39 mL of 0.01234 M Ce4+ for complete oxidation to Fe3+ (Ce4+ + Fe2+ → Ce3+ + Fe3+).
Calculate the weight percent of Cl in the original sample.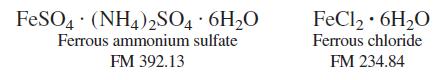
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





