Managing a salt-water aquarium. A tank at the New Jersey State Aquarium has a volume of 2.9
Question:
Managing a salt-water aquarium. A tank at the New Jersey State Aquarium has a volume of 2.9 million liters. Bacteria are used to remove nitrate that would otherwise build up to toxic levels. Aquarium water is first pumped into a 2 700-L deaeration tank containing bacteria that consume O2 in the presence of added methanol: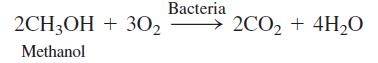
Anoxic (deoxygenated) water from the deaeration tank flows into a 1 500-L denitrification reactor containing colonies of Pseudomonas bacteria in a porous medium. Methanol is injected continuously and nitrate is converted into nitrite and then into nitrogen: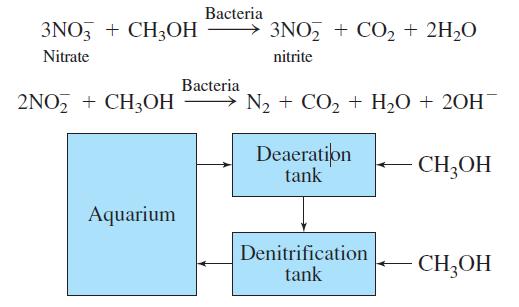
(a) Deaeration can be thought of as a slow, bacteria-mediated titration of O2 by CH3OH. The concentration of O2 in seawater at 24oC is 220 μM. How many liters of CH3OH (FM 32.04, density = 0.791 g/mL) are required by Reaction 1 for 2.9 million liters of aquarium water?
(b) Write the net reaction showing nitrate plus methanol going to nitrogen. How many liters of CH3OH are required by the net reaction for 2.9 million liters of aquarium water with a nitrate concentration of 8 100 μM?
(c) In addition to consuming methanol for Reactions 1 through 3, the bacteria require 30% more methanol for their own growth. What is the total volume of methanol required to denitrify 2.9 million liters of aquarium water?
Step by Step Answer:






