A barium sulfate precipitation titration described at the opening of this chapter is shown in the figure.
Question:
A barium sulfate precipitation titration described at the opening of this chapter is shown in the figure. The initial concentration of Cl- before adding BaCl2 was 0.000 19 M in 25 mL of aqueous extract of Martian soil. At the end point, when there is a sudden rise in Ba2+, [Cl-] = 0.009 ±M.
(a) Write the titration reaction.
(b) How many mmol of BaCl2 were required to reach the end point?
(c) How many mmol of SO24 - were contained in the 25 mL?
(d) If SO 24 - is derived from 1.0 g of soil, what is the wt% of SO24-in the soil?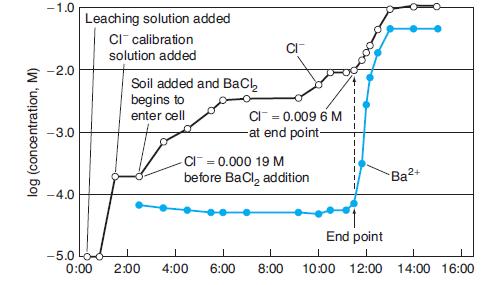
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





