A solution was prepared from 10.0 mL of 0.100 M cacodylic acid and 10.0 mL of 0.080
Question:
A solution was prepared from 10.0 mL of 0.100 M cacodylic acid and 10.0 mL of 0.080 0 M NaOH. To this mixture was added 1.00 mL of 1.27 × 1026 M morphine. Calling morphine B, calculate the fraction of morphine present in the form BH1.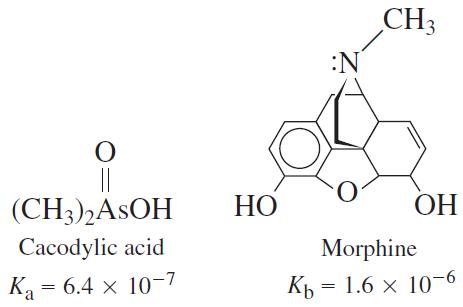
Transcribed Image Text:
CH3 :N || (CH3),ASOH НО ОН Cacodylic acid Morphine Ka = 6.4 x 10-7 Ка Къ — 1.6 х 10-6 Kp ||
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A solution was prepared by mixing 10.00 mL of unknown (X) with 5.00 mL of standard (S) containing 8.24 g S/mL and diluting the mixture to 50.0 mL. The measured signal quotient was (signal due to...
-
A solution was prepared from 1.023 g of the primary standard tris (Table 10 - 4) plus 99.367 g of water; 4.963 g of the solution were titrated with 5.262 g of aqueous HNO3 to reach the methyl red end...
-
A solution was prepared from 1.023 g of the primary standard tris (Table 10 - 4) plus 99.367 g of water; 4.963 g of the solution were titrated with 5.262g of aqueous HNO3 to reach the methyl red end...
-
Chuck, a single taxpayer, earns $86,000 in taxable income and $20,000 in interest from an investment in City of Heflin bonds. (Use the U.S. tax rate schedule.) Required: a. If Chuck earns an...
-
Use GSS 2010 to examine the relationship between respondents health (HEALTH) and social class (CLASS). Treat social class as the independent variable. a. Request the appropriate measures of...
-
A projectile is launched from point A with an initial velocity v0 of 120 ft/s at an angle ? with the vertical. Determine? (a) The distance d to the farthest point B on the hill that the projectile...
-
A company called CRW runs credit checks for a large number of banks and insurance companies. Credit history information is typed into computer files by trained administrative assistants. The company...
-
On January 1, 2014, Prince Corporation acquired 70% of the 100,000 outstanding voting shares of Song Limited for a cash consideration of $1,015,000. On that date, shares of Song Limited were trading...
-
****PLEASE SHOW ALL WORK AND CALCULATIONS FOR QUESTION 10**** ****PLEASE SHOW ALL WORK AND CALCULATIONS FOR QUESTION 10**** Innovative Distribution Company A Total Cost Approach to Understanding...
-
Your parents have made you an offer you can't refuse. They're planning to give you part of your inheritance early. They've given you a choice. Option (a) They'll pay you $11,000 per year for each of...
-
Calculate H2A , HA- , and A 2- for cis-butenedioic acid at pH 1.00, 1.92, 6.00, 6.27, and 10.00.
-
Explain what is wrong with the following statement: At its isoelectric point, the charge on all molecules of a particular protein is 0.
-
Match the compound with the appropriate carbonyl IR absorption band: acyl chloride ........................ ~1800 and 1750 cm-1 acid anhydride ..................... ~1640 cm-1 ester...
-
2. See US Debt Clock and answer the following: (Hint: Take a screenshot of the Debt Clock) (2) A. What is the current US deficit and the total federal debt? (1) B What is the net interest...
-
Q. Is GDP per capita a good measure of a society's welfare? Why or why not? (150 Words)
-
On May 3, the Happy Company wrote off the $4,300 uncollectible account of its customer, A. Johnson. The entry or entries Happy makes to record the write off of the account on May 3 is: Allowance for...
-
Who is responsible for the financial statements and maintaining effective internal control over financial reporting? Where did you find this in the annual report? What accounting rules are required...
-
8. Chad owned an office building that was destroyed in a tornado. The adjusted basis of the building at the time was $890,000. After the deductible, Chad received an insurance check for $850,000. He...
-
Implement the following method to sort the rows in a twodimensional array. A new array is returned and the original array is intact.public static double[][] sortRows(double[][] m)Write a test program...
-
A Alkynes can be made by dehydrohalogenation of vinylic halides in a reaction that is essentially an E2 process. In studying the stereochemistry of this elimination, it was found that...
-
Consider the titration of 100.0 mL of 0.010 0 M Ce 4+ in 1 M HClO 4 by 0.040 0 M Cu+ to give Ce 3+ and Cu 2+ , using Pt and saturated Ag | AgCl electrodes to find the end point. (a) Write a balanced...
-
Calcium fluorapatite (Ca 10 (PO 4 ) 6 F 2 , FM 1 008.6) laser crystals were doped with chromium to improve their efficiency. It was suspected that the chromium could be in the +4 oxidation state. 1....
-
Primary-standard-grade arsenic(III) oxide (As 4 O 6 ) is a useful (but carcinogenic) reagent for standardizing oxidants including MnO 4 - and I-3. To standardize MnO 4 - , As 4 O 6 is dissolved in...
-
Current Attempt in Progress On July 3 1 , 2 0 2 2 , Crane Compary had a cash balance per books of $ 6 , 2 4 5 . 0 0 . The statement from Dakata State Bark on that date showed a balance of $ 7 , 7 9 5...
-
Cede & Co. expects its EBIT to be $89,000 every year forever. The firm can borrow at 5 percent. Cede currently has no debt, and its cost of equity is 10 percent. If the tax rate is 35 percent, what...
-
In the Marriott example, one discussion point considered when a firm might use a single hurtle rather than different divisional or business unit rates. When a single rate is used and the divisions...

Study smarter with the SolutionInn App


