Color Plate 4 shows how the color of the acid-base indicator bromocresol green (H 2 BG) changes
Question:
Color Plate 4 shows how the color of the acid-base indicator bromocresol green (H2BG) changes as NaCl is added to an aqueous solution of (H+)(HBG-). Explain why the color changes from pale green to pale blue as NaCl is added.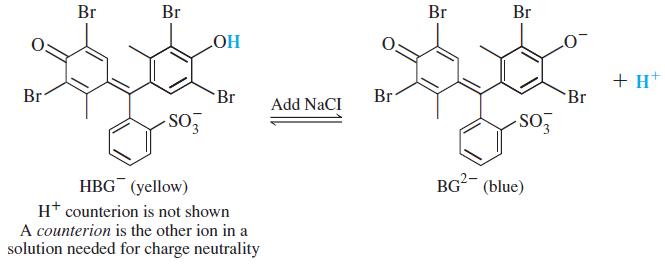
Transcribed Image Text:
Br Br Br Br Но + H* Br Br Br Br Add NaCI -SO BG (blue) HBG (yellow) H* counterion is not shown A counterion is the other ion in a solution needed for charge neutrality
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 87% (8 reviews)
On addition of NaCl the ionisabl...View the full answer

Answered By

Jyoti Chahal
I have done my graduation in Science from maharshi dayanand University, rohtak. I got 64.6% marks in bsc and masters in chemistry from Maharshi dayanand University with 64.5% marks. After that I did bachelor of education in science with passing marks of 69.4% from same University. I have given home tution also . Currently I am a subject matter expert on Transtutor.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The table in Figure 2.1 shows how the terms of the growth function for our dishwashing example are related to one another as n grows. Write a program that will create such a table for any given...
-
Heat is added to an ideal gas maintained at constant volume. Is it possible for the temperature of the gas to remain constant in this process? Explain.
-
Heat is added to an object initially at 30C, increasing its temperature to 80C. a. What is the temperature change of the object in Fahrenheit degrees? b. What is the temperature change of the object...
-
The maker of a $10,000, 8%, 90-day note receivable failed to pay the note on the due date of June 30. What accounts should be debited and credited by the payee to record the dishonored note...
-
Examine attitudes toward affirmative action based on two variables: AFFRMACT and DISCAFF. AFFRMACT measures respondents' support of preferential hiring and promotion of blacks (a higher score...
-
The motion of a particle is defined by the relation x = t3 (t 2)2 where x and t are expressed in meters and seconds, respectively. Determine (a) When the acceleration is zero, (b) The position and...
-
1. Why are you reading this book? What is your interest in culture?
-
Consider the following condensed financial statements of Secure Life, Inc. The companys target rate of return is 12%. Requirements 1. Calculate the companys ROI. Round all of your answers to four...
-
An insurance company has liabilities of 5 million due in 11 years' time and 13 million due in 17 years' time. The assets of the company consist of two zero-coupon bonds, one paying X million in 7...
-
Question: UCS leased equipment from PLS on January 1, 2024. PLS paid $625,483 for the equipment. The equipment's fair value is also $625,483. The lease term is 3 years, with semiannual periodic lease...
-
Use Equation 7-12 to reproduce the curves in Figure 7-3. Plot your results on a single graph. F(Kp = 8.3 x 10-17) %3D 14 12 Br (Kp = 5.0 x 10-13) %D 10 CF (K = 1.8 x 10-10) sp -Br 2 10 20 30 40 50 60...
-
A 40.0-mL solution of 0.040 0 M Hg 2 (NO 3 ) 2 was titrated with 60.0 mL of 0.100 M KI to precipitate Hg 2 I 2 (K sp = 4.6 10 -29 ). (a) Show that 32.0 mL of KI are needed to reach the equivalence...
-
Find the measure of the red arc or chord in C. A B C 75 E D
-
What are knowledge or innovation workers? What are the key elements of professional practice, work environment and work design needed to support the productivity and creativity of knowledge or...
-
What problem does this concept solve or what pain does it alleviate and how compelling is the problem? 2. Who is your specific target customer? 3. How do they currently meet this need for themselves...
-
Consider a person standing in a room where the average wall temperature is 20 C. This person is trying to reach the "thermal comfort" by adjusting the A/C air temperature. Find out the appropriate...
-
If WHO, the World Health Organization,defines health as a state of completephysical, mental and social well-being and not merely the absenceof disease and infirmity (WHO, 2011)and wellness is...
-
As a manager, you want to find a way to motivate Nate and increase his engagement and job satisfaction in the workplace. Drawing upon a behavioral theory of motivation, discuss how you, as a manager,...
-
The W-2 income of Sandra, a single taxpayer, was $88,793. Using the tax tables, determine Sandras tax liability.
-
A 6-lb shell moving with a velocity ?? v0k explodes at point D into three fragments which hit the vertical wall at the points indicated. Fragments A, B, and C hit the wall 0.010 s, 0.018 s, and 0.012...
-
Why do glass pH electrodes tend to indicate a pH lower than the actual pH in strongly basic solution?
-
Suppose that the Ag | AgCl outer electrode in Figure 14-11 is filled with 0.1 M NaCl instead of saturated KCl. Suppose that the electrode is calibrated in a dilute buffer containing 0.1 M KCl at pH...
-
(a) When the difference in pH across the membrane of a glass electrode at 25C is 4.63 pH units, how much voltage is generated by the pH gradient? (b) What would the voltage be for the same pH...
-
QUESTION 3 A business owns seven flats rented out to staff at R500 per month. All flats were tenanted Ist january 21 months rent was in arrears and as at 31st December 14 months' rent wa Identify the...
-
1. 2. 3. Select the Tables sheet, select cells A6:B10, and create range names using the Create from Selection button [Formulas tab, Defined Names group]. Select cells B1:F2 and click the Name box....
-
Tropical Rainwear issues 3,000 shares of its $18 par value preferred stock for cash at $20 per share. Record the issuance of the preferred shares. (If no entry is required for a particular...

Study smarter with the SolutionInn App


