Dry primary-standard-grade potassium hydrogen phthalate for 1 h at 110 o C and store it in a
Question:
Dry primary-standard-grade potassium hydrogen phthalate for 1 h at 110oC and store it in a desiccator.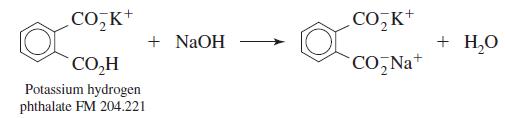
Transcribed Image Text:
CO,K+ CO, K+ + NaOH + H,0 CO,H CO, Na+ Potassium hydrogen phthalate FM 204.221
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)

Answered By

Akash Bongane
“I like to encourage students to ask questions and really understand subjects. I find non-traditional methods, like hands-on learning, to be particularly effective.”
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Buoyancy correction factor: mtrue/mread = 1.000 3244] [Interpolated density of water at 23.3 C: 0.997 468 9 g/mL] [HCl concentration at 20 C: 0.102343 M] Reagents: The molar mass and density of...
-
Potassium hydrogen phthalate is a primary standard used to measure the concentration of NaOH solutions. Find the true mass of potassium hydrogen phthalate (density = 1.636 g/mL) if the mass weighed...
-
Potassium hydrogen phthalate (KHP) is often used as a primary standard in acid-base titration. If 19.15 mL of NaOH is required to neutralize 0.442 g of KHP, what is the concentration of the NaOH?
-
Write a film script using this title "The arrival". THE ARRIVAL' WRITE A FILM SCRIPT USING THIS, TITLE DUMBFOUND: ASTONISH, AMAZE E62-67 SIMILE A METAPHOR ALLITERATION LIST OF THREE A LINE FROM A...
-
Segregation in schools appears to be increasing, due in part to class (and racial) separation between public and private schools. The table below summarizes the percentage of students who are white...
-
Racing cars A and B are traveling on circular portions of a race track. At the instant shown, the speed of A is decreasing at the e rate of 8m/s2, and the speed of B is increasing at the rate of...
-
Use the appropriate pattern-analysis rules to determine whether the process being monitored by the control chart shown on page 696 is under the influence of special causes of variation.
-
The auditor has completed her work on the financial statements of Leslie Kwok Inc. (LKI) for the year ended 31 December 20X7. The auditor signed her audit opinion on 5 March 20X8; LKIs board of...
-
Smithen Company, a wholesale distributor, has been operating for only a few months. The company sells three products- sinks, mirrors, and vanities. Budgeted sales by product and in total for the...
-
Aubrae and Tylor Williamson began operations of their furniture repair shop (Furniture Refinishers, Inc.) on January 1, 2019. The annual reporting period ends December 31. The trial balance on...
-
The inside cover of this book tells us that 8.2 mL of 37 wt% HCl should be added to 1 L of water to produce 0.1 M HCl. Prepare this solution in a capped polyethylene bottle, using a graduated...
-
Boil 1 L of water for 5 min to expel CO 2 . Pour the water into a polyethylene bottle, which should be tightly capped whenever possible. Calculate the volume of 50 wt% NaOH needed (5.3 mL) to produce...
-
If your course involves exercises with a CFD code, study the manual to determine which of the projection schemes discussed in Section 10.4 (SIMPLE, SIMPLEC, SIMPLER, PISO) are implemented. Are there...
-
Dr. Powers operates a single-provider family medical practice. One medical assistant handles appointments, basic bookkeeping functions, and assists with medical records. Two additional medical...
-
Quiz 6 Fall 2019 - MGCR-211-001/002/003 edugen.wileyplus.com WileyPLUS Financial Accounting, Seventh Canadian Edition by Kimmel, Weygandt, Kieso, Trenholm, Irvine, and Burnley Help | System...
-
In Exercises 21-24, use these results from the "1-Panel-THC" test for marijuana use, which is provided by the company Drug Test Success: Among 143 subjects with positive test results, there are 24...
-
4 Listen Using the DCF approach yields the value of the company as a whole. How would one refine this to determine the value of a share of stock? 1) Divide the company value by total assets. 2)...
-
The "is" or "is not" test established in McPhail v. Doulton (1971) for discretionary trusts creates more problems than it resolves.' Critically evaluate this statement. requirement Table of content...
-
Write a program that generates a 6-by-6 two-dimensional matrix filled with 0s and 1s, displays the matrix, and checks if every row and every column have an even number of 1s.
-
A simple random sample of 220 university students were asked what pasta they usually order and with which sauce. The preferences of these respondents are summarised below: Sauce Bolognese Pasta...
-
Iodometric analysis of high-temperature superconductor. The procedure in Box 15-3 was carried out to find the effective copper oxidation state, and therefore the number of oxygen atoms, in the...
-
Here is a description of an analytical procedure for superconductors containing unknown quantities of Cu(I), Cu(II), Cu(III), and peroxide (O 2 2- ): 32 "The possible trivalent copper and/or...
-
Li 1 + y CoO 2 is an anode for lithium batteries. Cobalt is present as a mixture of Co(III) and Co(II). Most preparations also contain inert lithium salts and moisture. To find the stoichiometry, Co...
-
C: The sor at the poopecin 0ieund to twe oxind places)
-
What information may an Appeals Officer not consider when reviewing a taxpayer's case? Select one: a. The cost involved for the IRS to hire an expert witness for litigation. b. Litigation hazards...
-
Carla Vista Cart Inc. has the following information for 2026 : The rate of return on assets Carla Vista Cart Inc. is 81.06%17.27%30.58%14.00%

Study smarter with the SolutionInn App


