Why do the liquid junctions potentials 0.1 M HCl | 0.1 M KCl and 0.1 M NaOH
Question:
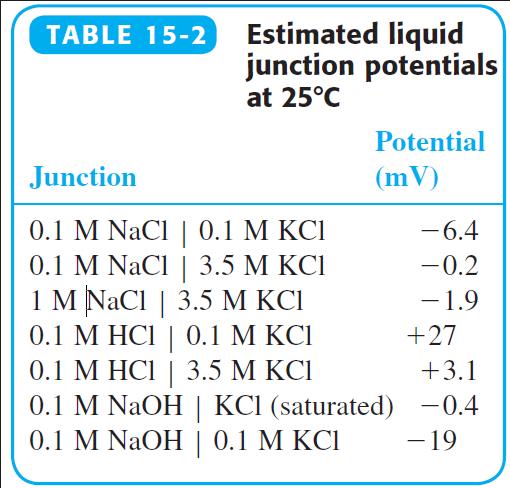
Why do the liquid junctions potentials 0.1 M HCl | 0.1 M KCl and 0.1 M NaOH | 0.1 M KCl have opposite signs in Table 15-2? Why is the junction potential for 0.1 M NaOH | 0.1 M KCl so much more negative than 0.1 M NaOH | KCl (saturated)?
Transcribed Image Text:
TABLE 15-2 Estimated liquid junction potentials at 25°C Potential Junction (mV) 0.1 M NaCl | 0.1 M KCI 0.1 M NaCl | 3.5 M KCI 1 M NaCl | 3.5 M KCI 0.1 M HCI | 0.1 M KCI 0.1 M HCI | 3.5 M KCI 0.1 M NaOH | KCI (saturated) -0.4 0.1 M NaOH | 0.1 M KCI -6.4 | -0.2 -1.9 | +27 +3.1 -19
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
Answer The liquid junction potentials 01 M HCl 01 M KCl and 01 M NaOH 01 M KCl have opposite signs b...View the full answer

Answered By

Danish Ahmad
I have good command on biology as well chemistry from my school days. As I have prepared for NEET and appeared in NEET 2019. I get enrolled in bachelor of pharmacy in year 2019 and now I have good command on biology(cell biology, genetics, cardiology, pharmacology, human physiology, biotechnology, microbiology, biochemistry etc) and chemistry(analytical chemistry, medicinal chemistry, physical, inorganic and organic chemistry) as well as other subjects of pharmacy also.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Why are salespeople so much more likely than other kinds of workers to be paid on a piece rate (i.e., on commission)? What is it about the kind of work they do that makes the high-commission +...
-
Why are supply shocks so much harder than demand shocks for monetary policy to adjust to? Use the grid below to show your answer to this question.
-
Why do managers have so much difficulty managing their time?
-
Consider a low-pass signal with a bandwidth of 3 kHz. A linear delta modulation system, with step size = 0.1V, is used to process this signal at a sampling rate ten times the Nyquist rate. (a)...
-
The chairperson of the board of directors at your firm asks for advice on SWP. What would you say?
-
Determine the force in each member of the Pratt bridge truss shown. State whether each member is in tension or compression. -3 -31 -3 - 6 kN 6 kN 6 kN
-
Suppose the data in Exercise 7.99 are based on independent random samples. a. Do the data provide sufficient evidence to indicate a difference between the mean strengths for the two types of shocks?...
-
The information in the table is from the statement of cash flows for a company at four different points in time (A, B, C, and D). Negative values are presented in parentheses. InstructionsFor each...
-
You would like to estimate the weighted average cost of capital for a new airline business. Based on its industry asset beta, you have already estimated an unlevered cost of capital for the firm of 8...
-
how would you alter the table structure? What data redundancies do you detect? Give example of how could those redundancies lead to data anomalies? How many records does the table contain? How many...
-
Consider the cell S.C.E. i cell solution | Pt(s), whose voltage is 20.126 V. The cell solution contains 2.00 mmol of Fe(NH 4 ) 2 (SO 4 ) 2 , 1.00 mmol of FeCl 3 , 4.00 mmol of Na 2 EDTA, and lots of...
-
The selectivity coefficient, KPot Li + , H + , for a Li + ion-selective electrode is 4 10 -4 . When this electrode is placed in 3.44 10 -4 M Li + solution at pH 7.2, the potential is 20.333 V...
-
Conduct a SWOT (Strength, Weakness, Opportunity, and Threat) analysis for the type of beverage you have selected, and for your company overall. As you work on the assignment, consider why you have...
-
Some people jump at the chance to be a change agent while others run from the role. Why is this the case? What are some characteristics of successful change agentry? These factors should refer to the...
-
An S corporation with $50,000 of earnings and profits owns rental real estate and has interest income producing investments. For the last two years, 50% of its gross receipts came from passive...
-
How has Biden Lowered premiums and out of pocket costs for millions of Americans?
-
Product costs using activity rates Body-Solid Inc. manufactures elliptical exercise machines and treadmills. The products are prouced in its Fabrication and Assembly production departments. In...
-
How to start an essay on the multigenerational workforce and your experiences working with each of the generations. Begin your essay with an introduction that outlines the current generations in the...
-
In 2017 Jessica bought a new heavy truck for $45,000 to use 80% for her sole proprietorship. Total miles driven include 12,000 in 2017, 14,500 in 2018, and 13,000 in 2019. a. If Jessica uses the...
-
Drainee purchases direct materials each month. Its payment history shows that 65% is paid in the month of purchase with the remaining balance paid the month after purchase. Prepare a cash payment...
-
(a) Following the example of ammonia in Section 7-5, write the equilibria and charge and mass balances needed to find the composition of 0.01 M sodium acetate, which you should abbreviate as Na+A-....
-
Calculate the ionic strength of (a) 0.0087 M KOH and (b) 0.0002 M La(IO3)3 (assuming complete dissociation at this low concentration and no hydrolysis reaction to make LaOH2+).
-
Find the activity coefficient of each ion at the indicated ionic strength: (a) SO24- ( = 0.01 M) (b) Sc3+ ( = 0.005 M) (c) Eu3+ ( = 0.1 M) (d) (CH3CH2)3NH+ ( = 0.05 M)
-
Al preparar el estado de resultados pro forma, cules de las siguientes partidas se deducen de las utilidades brutas para llegar a las ganancias despus de impuestos? Pregunta de seleccin mltiple....
-
Lawson Inc. is expanding its manufacturing plant, which requires an investment of $4 million in new equipment and plant modifications. Lawson's sales are expected to increase by $3 million per year...
-
20 On January 1, Year 1, X Company purchased equipment for $80,000. The company estimates that the equipment will have a useful life of 10 years and a residual value of $5,000. X Company depreciates...

Study smarter with the SolutionInn App


