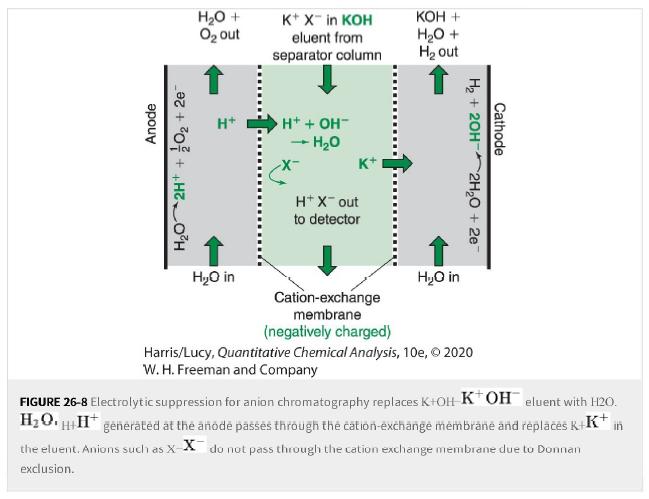
a. The suppressor in Figure 26-8 enables low parts per billion conductivity detection for anions such as
Question:
a. The suppressor in Figure 26-8 enables low parts per billion conductivity detection for anions such as Cl-Cl- and Br-,Br-. but very poor detection limits for anions such as CN-CN- and borate. Explain why.
b. Mixtures of sodium carbonate and bicarbonate can be used as eluent in suppressed-ion anion chromatography. Detection limits are poorer than with hydroxide eluent due to a higher background conductivity. Explain why.
Figure 26-8
Transcribed Image Text:
Anode H₂O 2H+ + O₂ +2e H₂O + O₂ out H+ H₂O in K+ X™ in KOH eluent from separator column H+ + OH™ + H₂O K+ H+ X-out to detector KOH + H₂O + H₂ out H₂O in H₂ + 2OH- Cathode 2H₂O + 2e Cation-exchange membrane (negatively charged) Harris/Lucy, Quantitative Chemical Analysis, 10e, © 2020 W. H. Freeman and Company FIGURE 26-8 Electrolytic suppression for anion chromatography replaces K+OH KOH eluent with H2O. H₂OH+H* generated at the anode passes through the cation-exchange membrane and replaces K+K+ in the eluent. Anions such as X-X do not pass through the cation exchange membrane due to Donnan exclusion.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (11 reviews)
Solution a The suppressor in Figure 268 enables low parts per billion conductiv...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Quantitative Chemical Analysis
ISBN: 9781319164300
10th Edition
Authors: Daniel C. Harris, Charles A. Lucy
Question Posted:
Students also viewed these Sciences questions
-
The carbonate ion (CO32-) can act as either a monodentate or a bidentate ligand. Draw a picture of CO32- coordinating to a metal ion as a bidentate and as a monodentate ligand. The carbonate ion can...
-
Explain why hydroxide ion catalyzes the reaction of piperidine with 2,4-dinitroanisole, but has no effect on the reaction of piperidine with 1-chloro-2,4-dinitrobenzene. piperidine
-
Why are some countries today much poorer than other countries? Are todays poor countries destined to always be poorer than todays rich countries? If so, explain why. If not, explain how todays poor...
-
Please show your work D To be a valid route, it must consist of a sequence of valid move. Each valid move is either going to the right for one block or going down for 1 block. T d) How many valid...
-
For the conditions of Exercise 12 and the data in Table 11.2, carry out a test of the following hypotheses: H0: 1 = 0, H1: 1 = 0.
-
a. In parallel columns, list the accounts that would be debited and credited for each of the following unrelated transactions: (1) Provided services for cash. (2) Recognized accrued salaries at the...
-
(e) Obtain predicted values of fibre diameter for each treatment at representative times and make a graphical display of the relationship between these variables.
-
A vertical aerial photograph reveals a tall building. The foot of one corner of the building has (x, y) coordinates (30.5, 62.0) (both measured in mm from the lower left-hand corner of the negative),...
-
How to find Subordinated debentures+Minority interest in consolidated (for suncorp bank australia)
-
If the redox potential for a soil at pH 6 is near zero, write two reactions that you would expect to take place. How would the presence of a great deal of nitrate compounds affect the occurrence of...
-
Conductivity and contactless conductivity detectors were developed for suppressed capillary ion chromatography. Observed peak heights in millivolts for bromide standards are in the table. a. Use...
-
Low iron concentration (as low as 0.02 nM 0.02 nM) in the open ocean limits phytoplankton growth. Preconcentration is required to determine such low concentrations. Trace Fe3+Fe 3+ from a large...
-
Purchasing Power Parity. Define the two forms of purchasing power parity, absolute and relative.
-
On a dreary morning in May 1995, Paul found himself sitting on the floor of the hallway, crouched against the cold wall, feeling dejected and desperate. The bustling cacophony of the people in nearby...
-
Two speakers S1 and S2 are at a distance from each other. Point Q is located at y = 2.1 m above loudspeaker S2 while point P is located at x = 4.1 m in front of loudspeaker S1. The two loudspeakers...
-
Q1 Go to the Office of the Superintendent of Financial Institutions (OSFI) and find data (as of December 31, 2020) for all domestic banks on total liabilities, total deposits, and residual of assets...
-
Explain your viewpoint/philosophy on the Christian's responsibility to demonstrate wise stewardship of higher education resources. How should a believer handle the institution's finances? As you...
-
TranscribedText: 4. DQ 5. Create your initial post on the DQ 5 Discussion Board in response to the following: e There are many different opinions about how media, specifically TV and the Internet,...
-
Consider again the insect in Problem 58. If the insect moves the 10-cm distance in 0.50 s, what is the magnitude of the Doppler shift in the bats reflected sound wave? Assume the insect is moving...
-
The executor of Gina Purcells estate has recorded the following information: Assets discovered at death (at fair value): Cash . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ....
-
State whether the errors in (a) - (d) are random or systematic: (a) A 25-mL transfer pipet consistently delivers 25.031 0.009 mL. (b) A 10 - mL buret consistently delivers 1.98 0.01 mL when drained...
-
State whether the errors in (a) - (d) are random or systematic: (a) A 25-mL transfer pipet consistently delivers 25.031 0.009 mL. (b) A 10 - mL buret consistently delivers 1.98 0.01 mL when drained...
-
Cheryl, Cynthia, Carmen, and Chastity shot the targets below at Girl Scout camp. Match each target with the proper description. (a) Accurate and precise (b) Accurate but not precise (c) Precise but...
-
Which of the following is a limitation of both return on investment and residual income? A. Favors large units. B. There is disincentive for high return on investment units to invest. C. Can lead to...
-
For anOld Country Links, Incorporated, produces sausages in three production departments Mixing , Casing and Curing, and Packaging. In the Mixing Department, meats are prepared, ground and mixed with...
-
A manufacturing firm uses a predetermined manufacturing overhead rate to allocate overhead to individual jobs, based on machine hours required. At the beginning of 2 0 1 9 , the firm expected to...

Study smarter with the SolutionInn App


