Question: Conductivity and contactless conductivity detectors were developed for suppressed capillary ion chromatography. Observed peak heights in millivolts for bromide standards are in the table. a.
Conductivity and contactless conductivity detectors were developed for suppressed capillary ion chromatography. Observed peak heights in millivolts for bromide standards are in the table.
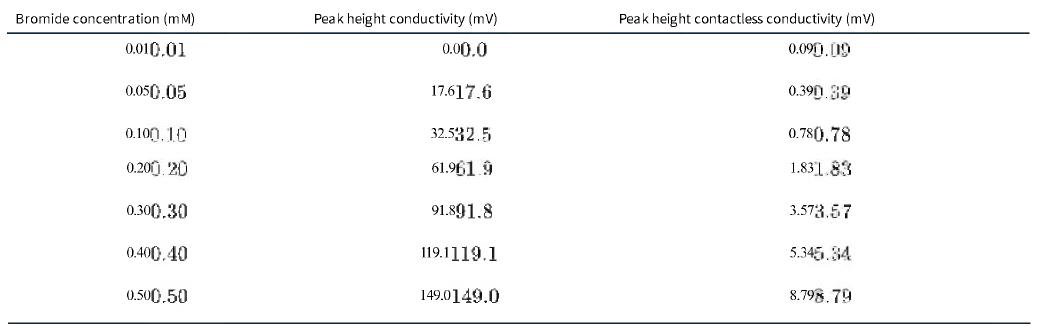
a. Use Excel to draw a graph of peak height for conductivity detection (column 2) versus Br-Br- concentration, and add a trendline. Write the equation for the line including standard uncertainties in the slope and intercept. Is the calibration linear?
b. Use Excel to graph peak height for contactless conductivity detection (column 3) versus Br-Br- concentration. Is the calibration linear? If not, what is the linear range for this calibration?
c. Fitting the contactless conductivity peak heights to a quadratic function yields y=29.84x2+1.906x+0.190 у=29.84x2 + 1.906x + 0.190 with negligible residuals and R2=0.996.R2 = 0.996. An unknown yields a peak height of 1.46 mV.1.46 mV. How much bromide is in the unknown?
d. Assuming the answer in (c) is the correct concentration, how much error would result if the linear calibrations in (b) had been used to determine the concentration of an unknown that yielded a peak height of 1.46 mV 1.46 mV?

Bromide concentration (mM) 0.010.01 0.050.05 0.100.10 0.200.20 0.300.30 0.400.40 0.500.50 Peak height conductivity (mv) 0.00.0 17.617.6 32.532.5 61.961.9 91.891.8 119.1119.1 149.0149.0 Peak height contactless conductivity (mv) 0.090.09 0.390.39 0.780.78 1.831.83 3.573.57 5.345.84 8.798.79
Step by Step Solution
3.45 Rating (174 Votes )
There are 3 Steps involved in it
Answer a The equation for the line is y 0068x 0502 with a slope uncertainty o... View full answer

Get step-by-step solutions from verified subject matter experts


