Question: a. Write the half-reactions for a H2-O2H 2 -O 2 fuel cell. Find the theoretical cell voltage at 25C 25C if PH2=1.0 bar, PH 2
a. Write the half-reactions for a H2-O2H2-O2 fuel cell. Find the theoretical cell voltage at 25°C 25°C if PH2=1.0 bar, PH2=1.0 bar, PO2=0.2 bar PO2=0.2 bar and [H+]=0.5 M[H+] = 0.5 M at both electrodes. (Real fuel cells operate at temperatures of 60° -1 000°C 60° -1 000°C and produce ~0.7 V.~0.7 V.)
b. If the cell is 70% 70% efficient at converting chemical energy into electrical energy, and a stack of cells produces 20 kW 20kW at 220 V, 220 V how many grams of H2H2 are consumed in an hour?
c. In the United States, engines are frequently rated in “horsepower.” Use Table 1-4 to convert 20 kW 20 kW to horsepower.
Table 1-4
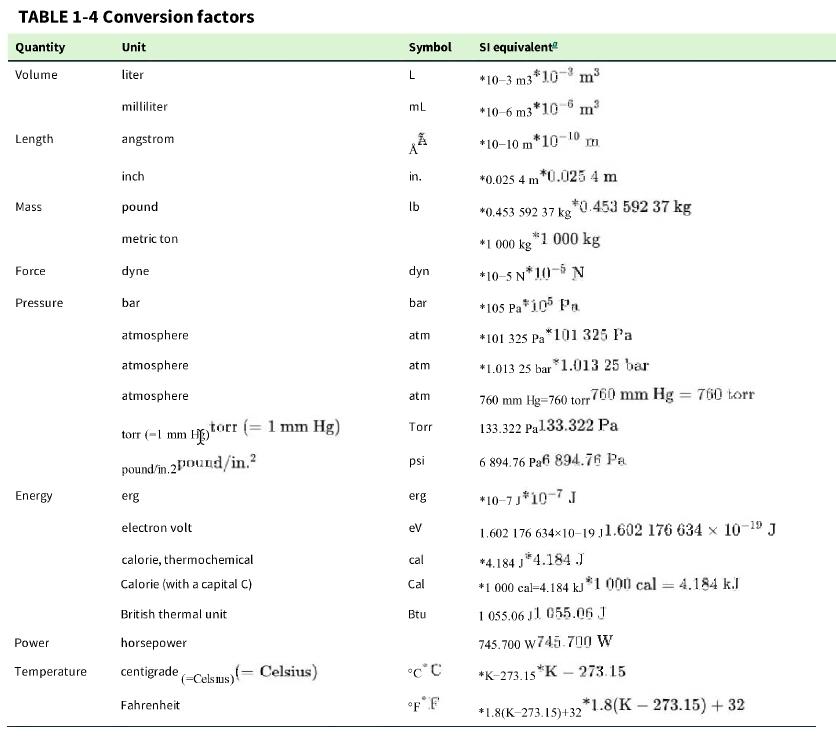
TABLE 1-4 Conversion factors Quantity Volume Length Mass Force Pressure Energy Unit liter Power milliliter angstrom inch pound metric ton dyne bar atmosphere atmosphere atmosphere torr (-1 mm H torr (= 1 mm Hg) pound/in.2 pound/in. erg electron volt calorie, thermochemical Calorie (with a capital C) British thermal unit horsepower Temperature centigrade Celsius) Fahrenheit Celsius) Symbol L mL A in. lb dyn bar atm atm atm Torr psi erg eV cal Cal Btu C C F F SI equivalent *10-3 m3*10-3 m *10-6 m3*106 m *10-10 m*10-10 m *0.025 4 m *0.025 4 m *0.453 592 37 kg *0.453 592 37 kg *1 000 kg *1 000 kg *10-5 N*10* N *105 Pa* 105 Pa *101 325 Pa *101 325 Pa *1.013 25 bar *1.013 25 bar 760 mm Hg-760 torr 760 mm Hg = 760 torr 133.322 Pa133.322 Pa 6 894.76 Pa6 894.76 Pa *10-7J*10-7 J 1.602 176 63410-19 11.602 176 634 x 10-9 J *4.184 J*4.184.J *1 000 cal-4.184 kJ *1 000 cal = 4.184 kJ 1055.06 J1 055.06 J 745.700 w745.700 W *K-273.15 *K-273.15 2*1.8(K-273.15) + 32 *1.8(K-273.15)+32
Step by Step Solution
3.32 Rating (167 Votes )
There are 3 Steps involved in it
a The halfreactions for a H2O2 fuel cell are At the cathode reduction O2 2H 2e H2O At the anode oxid... View full answer

Get step-by-step solutions from verified subject matter experts


