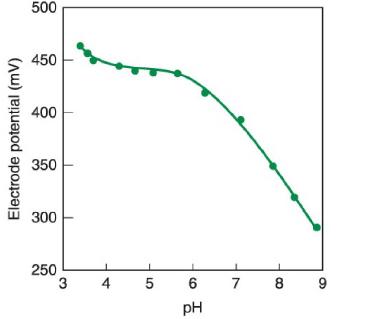
The figure shows the effect of pHpH on the response of a nitrite (NO2-) (NO - 2
Question:
The figure shows the effect of pHpH on the response of a nitrite (NO2-) (NO-2) ion-selective electrode immersed in 1 μM NaNO2+1 mM NaCLl. 1 μM NaNO2+1 mM NaCLl. The authors recommend using the flat region near pH5.5pH5.5 for measuring nitrite. To a first approximation, we expect the electrode to respond to [NO2-][NO-2] by the equation E=constant-(59 mV) log [NO2-].E = constant - (59 mV) log [NO-2].
a. Look up pKapKa for nitrous acid and explain the behavior of the curve at pH<4.pH < 4.
b. Suggest an explanation for the behavior at pH>6.pH > 6.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Quantitative Chemical Analysis
ISBN: 9781319164300
10th Edition
Authors: Daniel C. Harris, Charles A. Lucy
Question Posted:





