In Figure 22-14, the sucralose species with a nominal mass X=395 X=395 is [12C121H1816O835C13]-. [ 12 C
Question:
In Figure 22-14, the sucralose species with a nominal mass X=395 X=395 is [12C121H1816O835C13]-. [12C121H1816O835Cl3]-. X+1X + 1 arises from isotopologues containing one 13C,13C, one 2H,2H, or one 17O.17O. The predicted intensity at X+1X+1 is 12x1.08%+18x0.012%+8x0.038%=13.5% 12 x 1.08%+18 x 0.012%4-8 x 0.038%= 13.5% of X.X. Use factors in Table 22-2 to write the analogous equation for the intensity at X+2 X + 2 arising from just [13C212C101H1816O835C13]-[13C212C101H1816O835Cl3]- and [12C121H1818O116O735C13]-.[12C121H1818O116O735Cl3]-. Why is the intensity at X+2 X+2 so much greater than what you calculate?
Figure 22-14
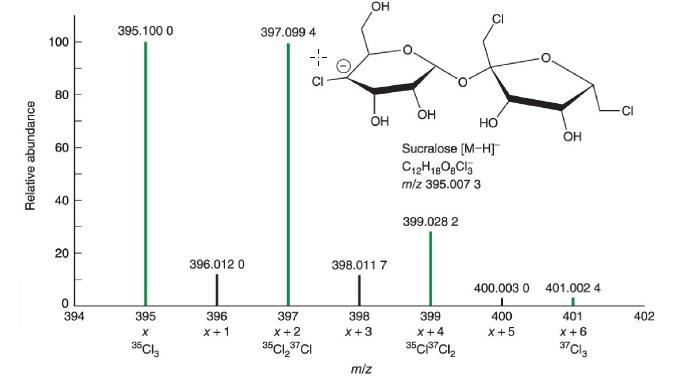
Table 22-2

Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Quantitative Chemical Analysis
ISBN: 9781319164300
10th Edition
Authors: Daniel C. Harris, Charles A. Lucy
Question Posted:





