Is it possible to precipitate 99.0% of 0.010 M Ce 3+ by adding oxalate (C 2 O
Question:
Is it possible to precipitate 99.0% of 0.010 M Ce3+ by adding oxalate (C2O42-) without precipitating 0.010 M Ca2+?
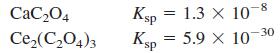
Transcribed Image Text:
CaC204 Ksp = 1.3 x 10-8 %3D Ce,(C,O4)3 Ksp 5.9 X 10-30
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Is it possible to make sound managerial decisions without business research? What advantages does research offer to the decision maker over seat-of-the-pants decision making?
-
Is it possible to design and enforce a law so that only honest citizens have access to guns?
-
Is it possible to use quantitative assessments of the organization's human resources to better link human resource management to firm strategy? Explain.
-
Selected accounts from the ledger of Restoration Arts for the fiscal year ended April 30, 2019, are as follows: Prepare a statement of owner's equity for the year. Apr 30 Doug Stone Capital Doug...
-
Mr. Z owns three homes. He lives in the San Francisco home full time. The other two he and his wife vacation in each year. He is considering replacing the house he has in Palm Springs, California,...
-
DE 10-8 At the beginning of 20X1. Vantas Airways purchased a used Boeing MD-11 aircraft at a cost of $36.000.000. Vantas expects the plane to remain useful for five years (5 million miles) and to...
-
Rank the stocks from best to worst (e.g., lowest P/E to highest P/E, lowest P/B to highest P/B, and so on for each ratio). Stock P/E P/B P/S PEG Profit margin Return on divided by equity divided by...
-
Gazarra Company is a very profitable small business. It has not, however, given much consideration to internal control. For example, in an attempt to keep clerical and office expenses to a minimum,...
-
Tannin Products Inc. prepared the following factory overhead cost budget for the Trim Department for July of the current year, during which it expected to use 8,000 hours for production: Variable...
-
1. Did the standards result in safer and more effective firefighting crews, or were they inadvertently keeping women out of a traditionally male job? 2. Was this a BFOQ? The ministry was challenged...
-
Is it possible to precipitate 99.0% of 0.010 M Ce 3+ by adding oxalate (C 2 O 4 2- ) without precipitating 0.010 M Ca 2+ ?
-
For a solution of Ni 2+ and ethylenediamine, the following equilibrium constants apply at 20C: Calculate the concentration of free Ni 2+ in a solution prepared by mixing 0.100 mol of en plus 1.00 mL...
-
Draw the Lewis structure and state the number of lone pairs on the central atom of each of the following molecules: (a) IF 5 ; (b) AsF 5 ; (c) H 2 SO 3 (S is the central atom and each H atom is...
-
Assume you have been given $400,000 CAD with access to all listed stocks, bonds, futures, and options worldwide. You can trade in options and futures, in combination with the underlying asset....
-
Charlene wrote a letter to Rachel offering to sell her car, a Proton Saga, for RM 60,000. The letter reached Rachel on 25. 11.2020. Rachel sent her letter of acceptance at 3 p.m. on the same day....
-
Data for the risk premium sensitivities (b, s, and h) as well as the beta coefficient for the CAPM of two companies are listed in the following table: Company b s h ERP SMBP HMLP Beta Alpha 1.1114...
-
Free-Response Questions 1. m Initial position eviribrA ARAL m Incline raised to 0 <0max pr A block of mass m is initially at rest on a rough board, which is initially horizontal on a tabletop. The...
-
A picture frame sits atop a bookshelf. When the bookshelf is bumped, the frame tumbles to the floor, landing after 0.64 s. How tall is the bookshelf?
-
Refer to the Human Factors (Dec. 1988) study of orientation clues, Exercise 9.6. Conduct a test to compare the proportions of subjects that fall in the three disk-orientation categories. Assume you...
-
5. Convert the following ERD to a relational model. SEATING RTABLE Seating ID Nbr of Guests Start TimeDate End TimeDate RTable Nbr RTable Nbr of Seats RTable Rating Uses EMPLOYEE Employee ID Emp...
-
Iminodiacetic acid forms 2:1 complexes with many metal ions: A 25.0 mL solution containing 0.120 M iminodiacetic acid buffered to pH 7.00 was titrated with 25.0 mL of 0.050 0 M Cu 2+ .Given that x2...
-
Iminodiacetic acid forms 2:1 complexes with many metal ions: A 25.0 mL solution containing 0.120 M iminodiacetic acid buffered to pH 7.00 was titrated with 25.0 mL of 0.050 0 M Cu 2+ .Given that x2...
-
What is the chelate effect?
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App



