Question
a. A reaction profile (not to scale!) for the reaction NO + CO 2 ? NO2 + CO is shown below: The value of the
a. A reaction profile (not to scale!) for the reaction NO + CO 2 ? NO2 + CO is shown below:
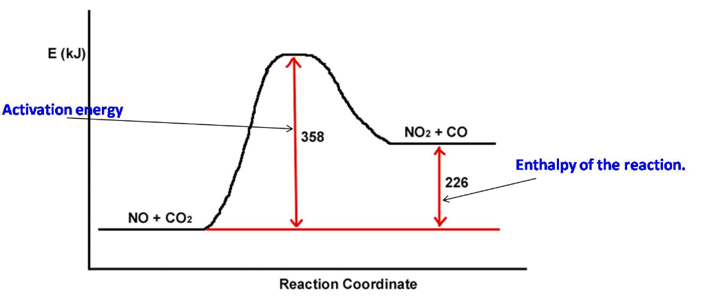
The value of the activation energy for this reaction is__________ kj and the value of ?E is ________kJ.
b. A reaction profile (not to scale!) for the reaction C 2 H 5 C 1 ?C 2 H 4 + HCI is shown below:
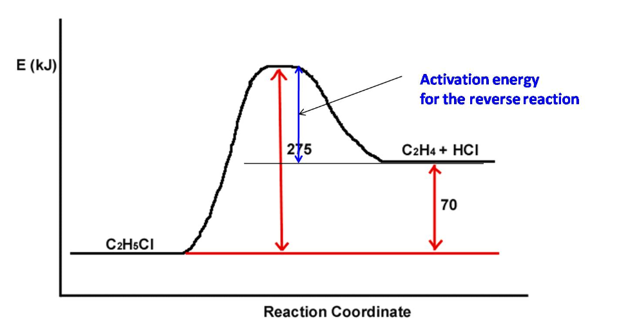
The reaction as written is _________
Assuming that the reaction is reversible, the value of the activation energy for the reverse reaction is____ kJ.
E (KJ) Activation energy NO + CO2 358 NO2 + CO Reaction Coordinate 226 Enthalpy of the reaction. E (KJ) C2H5CI 275 Activation energy for the reverse reaction C2H4 + HCI Reaction Coordinate 70
Step by Step Solution
3.32 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
a Activation energy for the reaction 358 K...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



