Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A chemist mixes solid AgCl. CuC12, and MgC12 in enough water to give a final volume of 50.0 mL. (a) With ions shown as spheres
A chemist mixes solid AgCl. CuC12, and MgC12 in enough water to give a final volume of 50.0 mL. (a) With ions shown as spheres and solvent molecules omitted for clarity, which scene best represents the resulting mixture? 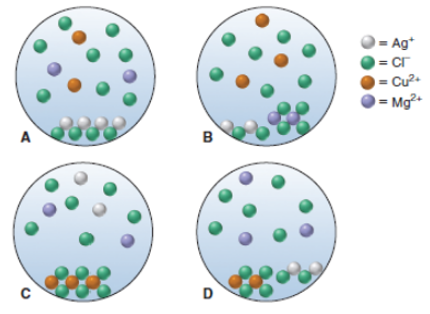
(b) If each sphere represents 5.0 ? 10 -3 mol of ions, what is the total concentration of dissolved (separated) ions?
(c) What is the total mass of solid?
A Bos B D of Ag+ =cr = Cu+ = Mg+
Step by Step Solution
★★★★★
3.28 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
Plan Check the solubility rules to see if the compounds are soluble or i...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
60946b216f29d_24825.pdf
180 KBs PDF File
60946b216f29d_24825.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


