Answered step by step
Verified Expert Solution
Question
1 Approved Answer
0.004 was wrong and so was 0.00367. I need help. The following table provides some information on carbon dioxide solubility in water. Sgas Pgas (mol
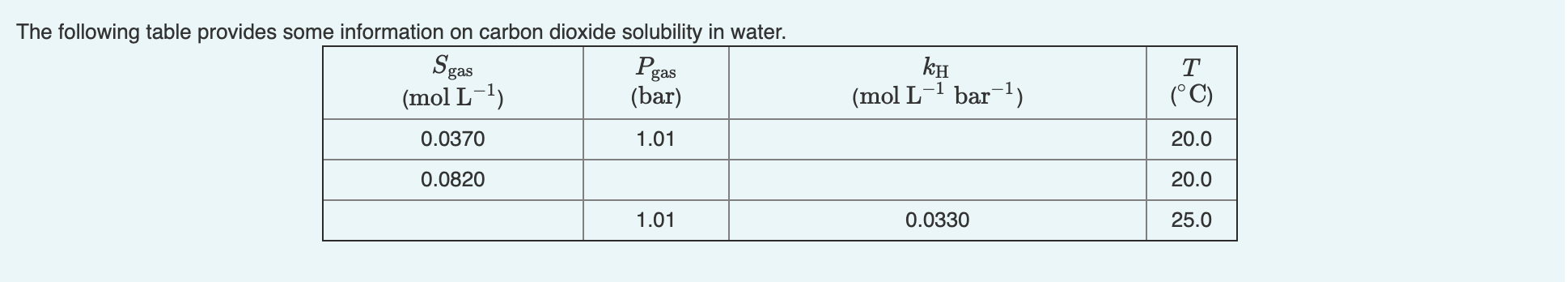
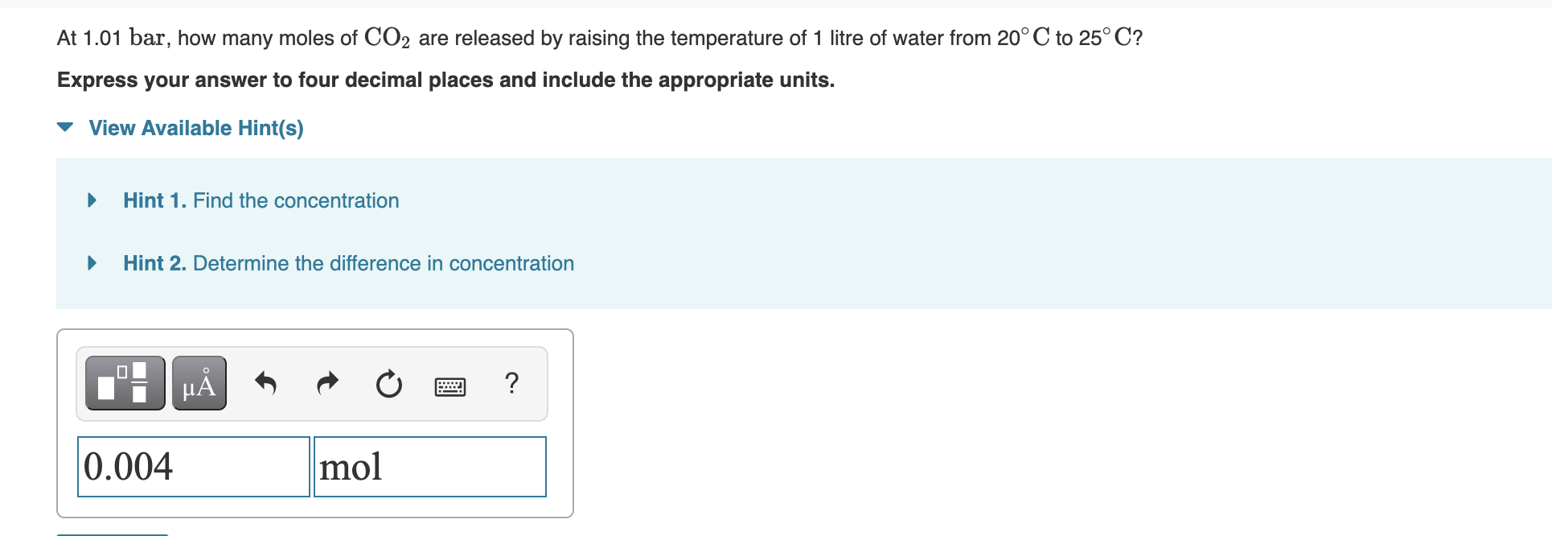
0.004 was wrong and so was 0.00367. I need help.
The following table provides some information on carbon dioxide solubility in water. Sgas Pgas (mol L-1) (mol L-1 bar-1) T (C) (bar) 0.0370 1.01 20.0 0.0820 20.0 1.01 0.0330 25.0 At 1.01 bar, how many moles of CO2 are released by raising the temperature of 1 litre of water from 20C to 25C? Express your answer to four decimal places and include the appropriate units. View Available Hint(s) Hint 1. Find the concentration Hint 2. Determine the difference in concentration ? 0.004 molStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


