Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. (40 pts) Citric acid (C6H8O7) is used in the preparation of many foods, pharmaceuticals, and personal care products. Although it can be recovered

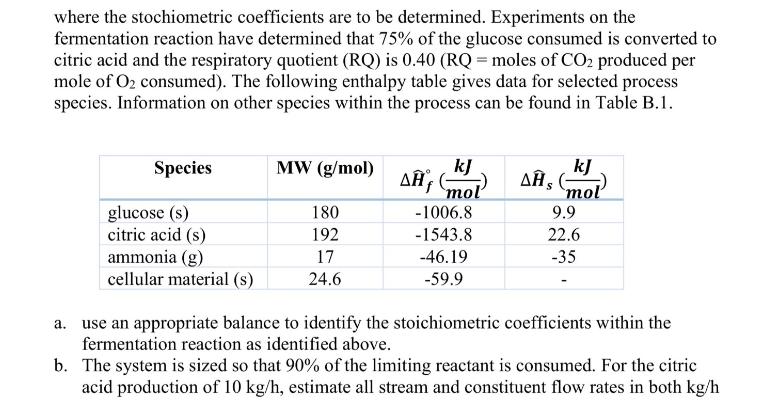

1. (40 pts) Citric acid (C6H8O7) is used in the preparation of many foods, pharmaceuticals, and personal care products. Although it can be recovered by concentration and crystallization from citrus juices, modern commercial production involves synthesis by fermentation of carbohydrates by Aspergillus niger. The process involves addition of the fungus to a fermenter along with carbohydrates, nutrients, water, and air that is bubbled through the fermentation broth. After the desired production of citric acid, the resultant fermentation liquor is filtered to remove the cell mass and other suspended solids followed by recovery and purification of citric acid by crystallization. As part of the evaluation of a proposed continuous fermentation process, you have been tasked with estimation of the required heating/cooling requirements for the fermenter designed to produce 10 kg/h of citric acid. Feed into the unit includes an aqueous solution that is 20 wt.% glucose, 0.4wt% ammonia, and the balance water. Air at 1.2 atm, saturated with water, is bubbled through fermenter providing a molar flowrate of oxygen twice that of glucose. One major stream leaving the fermenter is a gas stream at 1 atm containing nitrogen, the unreacted oxygen, carbon dioxide produced during the fermentation process. This gas stream is also saturated with water. The other major stream leaving the fermenter is a liquid fermentation liquor containing water, citric acid, unreacted glucose, and unreacted ammonia. All streams are assumed to exist at 25 C and the contents of the fermenter can be assumed to be well mixed due to the nature of air bubbling within the fermenter. The fermentation reaction is given as: C6H12O6 (aq) + a NH3(aq) + b O(9) C CH1.79N0.200.5 (s) + d CO2 + e HO (1) + f C6H8O7 (aq) where the stochiometric coefficients are to be determined. Experiments on the fermentation reaction have determined that 75% of the glucose consumed is converted to citric acid and the respiratory quotient (RQ) is 0.40 (RQ = moles of CO2 produced per mole of O2 consumed). The following enthalpy table gives data for selected process species. Information on other species within the process can be found in Table B.1. Species MW (g/mol) kJ kJ A mol , (mol glucose (s) citric acid (s) 180 -1006.8 9.9 192 -1543.8 22.6 ammonia (g) 17 -46.19 -35 cellular material (s) 24.6 -59.9 a. use an appropriate balance to identify the stoichiometric coefficients within the fermentation reaction as identified above. b. The system is sized so that 90% of the limiting reactant is consumed. For the citric acid production of 10 kg/h, estimate all stream and constituent flow rates in both kg/h and mol/h. What are the volumetric flow rates of air entering the fermenter and the off-gas stream (in L/h)? c. The heats of formation provided within the table indicate the phases of materials; however, the reaction is specified with substances within the aqueous phase. Show how Hess's law can be used to estimate the heat of formation in the aqueous phase based on the provided enthalpies for the gaseous and solid species. d. Estimate the rate at which heat must be added or removed from the fermenter (in kW).
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


