Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. A 60kmol/h liquid solution which is at 25C, contains 32mol percent ethylene glycol and 68mol percent water. This solution will be flash- distilled at
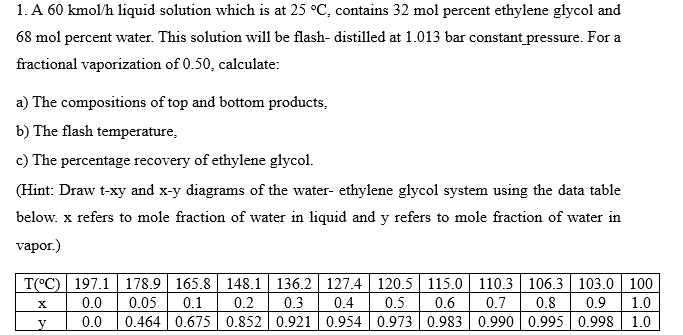 1. A 60kmol/h liquid solution which is at 25C, contains 32mol percent ethylene glycol and 68mol percent water. This solution will be flash- distilled at 1.013 bar constant_pressure. For a fractional vaporization of 0.50 , calculate: a) The compositions of top and bottom products, b) The flash temperature, c) The percentage recovery of ethylene glycol. (Hint: Draw t-xy and x-y diagrams of the water- ethylene glycol system using the data table below. x refers to mole fraction of water in liquid and y refers to mole fraction of water in vapor.)
1. A 60kmol/h liquid solution which is at 25C, contains 32mol percent ethylene glycol and 68mol percent water. This solution will be flash- distilled at 1.013 bar constant_pressure. For a fractional vaporization of 0.50 , calculate: a) The compositions of top and bottom products, b) The flash temperature, c) The percentage recovery of ethylene glycol. (Hint: Draw t-xy and x-y diagrams of the water- ethylene glycol system using the data table below. x refers to mole fraction of water in liquid and y refers to mole fraction of water in vapor.) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


