Answered step by step
Verified Expert Solution
Question
1 Approved Answer
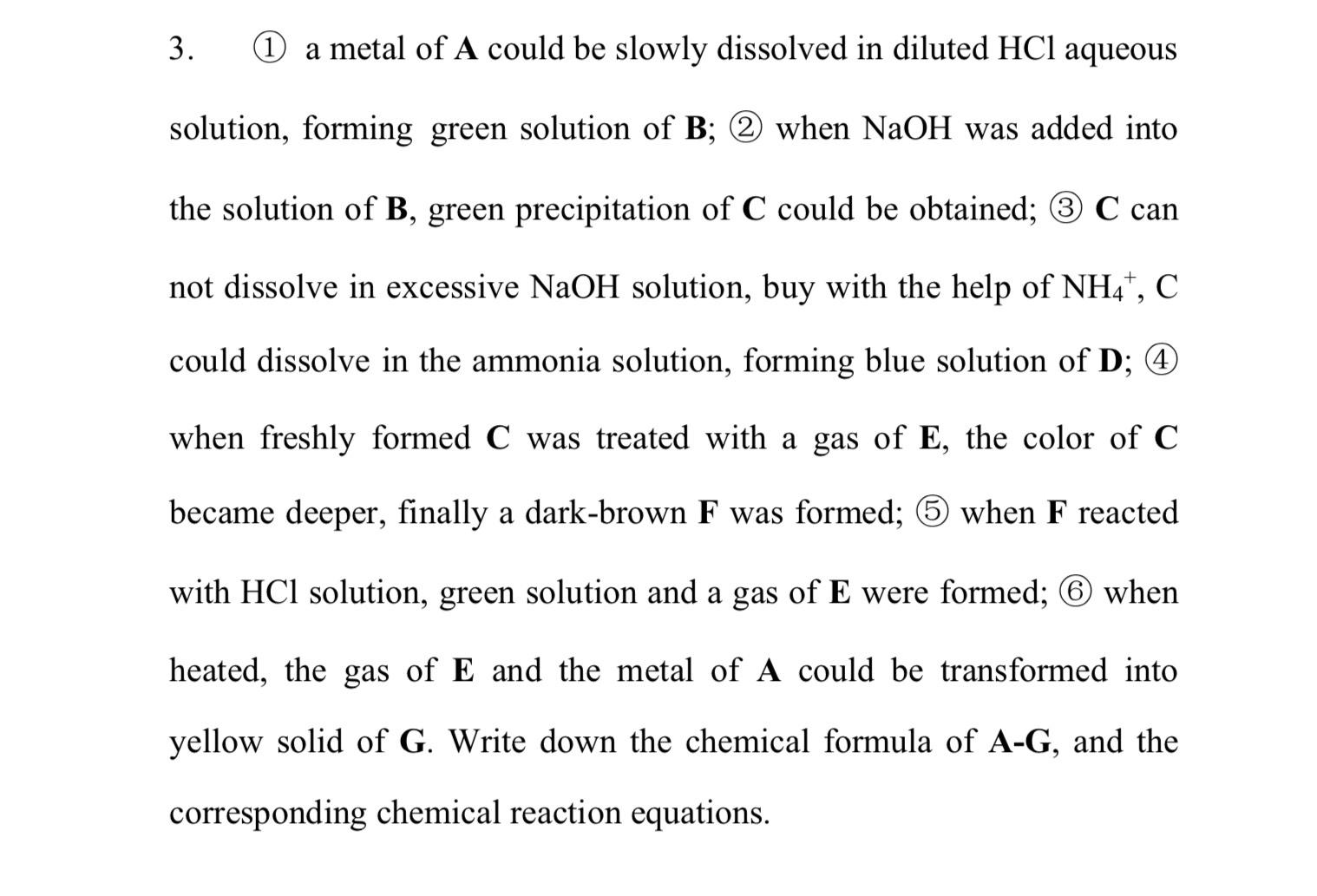
( 1 ) a metal of A could be slowly dissolved in diluted H C l aqueous solution, forming green solution of B ; (
a metal of A could be slowly dissolved in diluted aqueous solution, forming green solution of ; when NaOH was added into the solution of green precipitation of could be obtained; can not dissolve in excessive NaOH solution, buy with the help of could dissolve in the ammonia solution, forming blue solution of ; when freshly formed was treated with a gas of the color of became deeper, finally a darkbrown was formed; when reacted with solution, green solution and a gas of were formed; when heated, the gas of and the metal of A could be transformed into yellow solid of Write down the chemical formula of and the corresponding chemical reaction equations.Its about dblock transition metal elements
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


