Question
1. A student notices that the product at the end of the first reaction does not appear to be substantially viscous. The student carries on
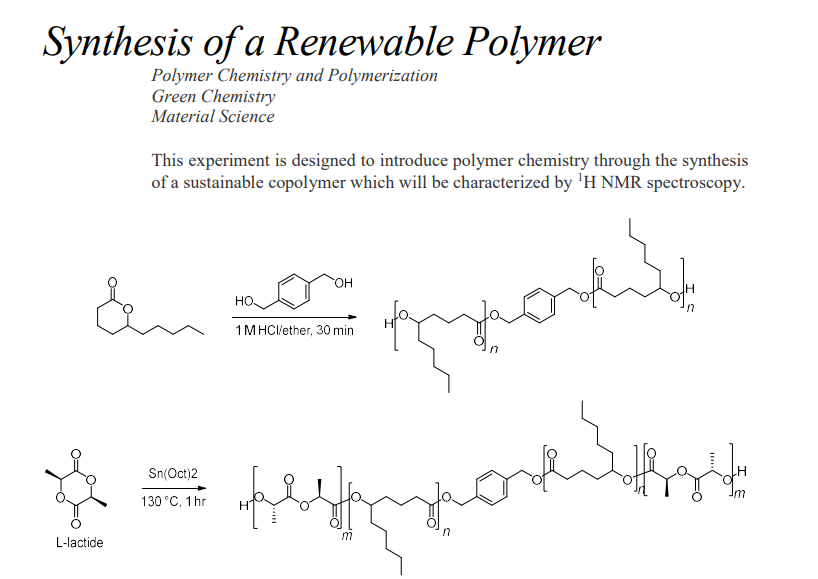
1. A student notices that the product at the end of the first reaction does not appear to be substantially viscous. The student carries on with the experiment, but does not obtain any precipitate after pouring the product into methanol. After performing a biphasic extraction, the student isolates an oily substance which is characterized by 1H NMR spectroscopy. After referencing the signal at 7.34 ppm to an integration of 4, the student finds that the signal at 4.86 ppm has an integration of about 12 and the signal at 5.16 ppm has an integration of 10. What can you conclude about the extent of the polymerization reaction for each of the steps? Suggest a possible contaminant or procedural errors that could have caused this problem.
2. A second student also does not obtain any precipitate. After a biphasic workup, the student characterizes the oily product obtained by 1H NMR spectroscopy. The signal at 4.86 ppm has an integration of 84 and the signal at 5.16 ppm has an integration of 9. What can you conclude about the extent of the polymerization reaction for each of the steps? Suggest a possible contaminant or procedural errors that could have caused this problem.
Synthesis of a Renewable Polymer Polymer Chemistry and Polymerization Green Chemistry Material Science This experiment is designed to introduce polymer chemistry through the synthesis of a sustainable copolymer which will be characterized by 1H NMR spectroscopy. 130C,1hrSn(Oct)2 L-lactideStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


