Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Application of Legendre transform on van der Waal gas: For monatomic van der Waal gas, the entropy is 3/21 N2a S (E,V,N) =
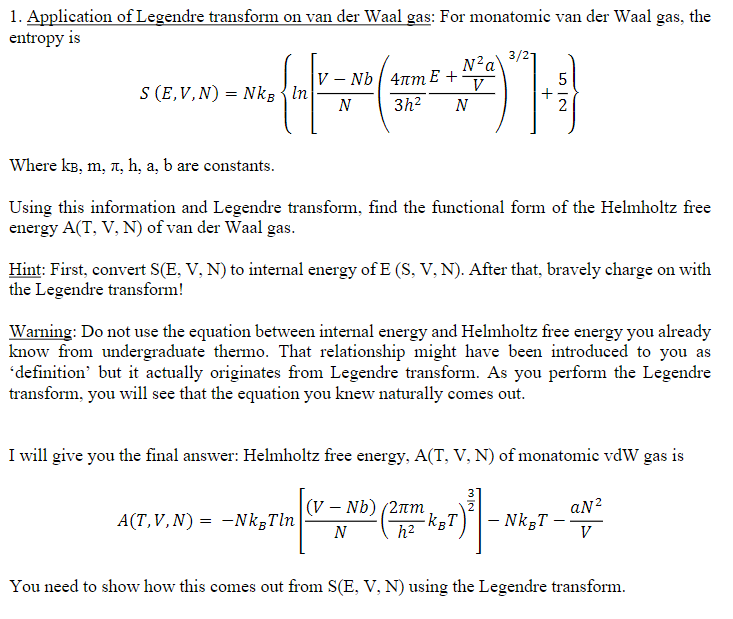
1. Application of Legendre transform on van der Waal gas: For monatomic van der Waal gas, the entropy is 3/21 N2a\ S (E,V,N) = Ng In V-Nb 4m E + N 3h2 N 5 V 2 Where KB, m, , h, a, b are constants. Using this information and Legendre transform, find the functional form of the Helmholtz free energy A(T, V, N) of van der Waal gas. Hint: First, convert S(E, V, N) to internal energy of E (S, V, N). After that, bravely charge on with the Legendre transform! Warning: Do not use the equation between internal energy and Helmholtz free energy you already know from undergraduate thermo. That relationship might have been introduced to you as 'definition' but it actually originates from Legendre transform. As you perform the Legendre transform, you will see that the equation you knew naturally comes out. I will give you the final answer: Helmholtz free energy, A(T, V, N) of monatomic vdW gas is = A(T,V,N) -NkTln [(V Nb) (2m N h .kBT - NkBT- - aN V You need to show how this comes out from S(E, V, N) using the Legendre transform.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


