Question
1. Calculate the bubble point temperature and vapor composition of a binary mixture with the following liquid composition: 20 mol% ethyl acetate (1) and
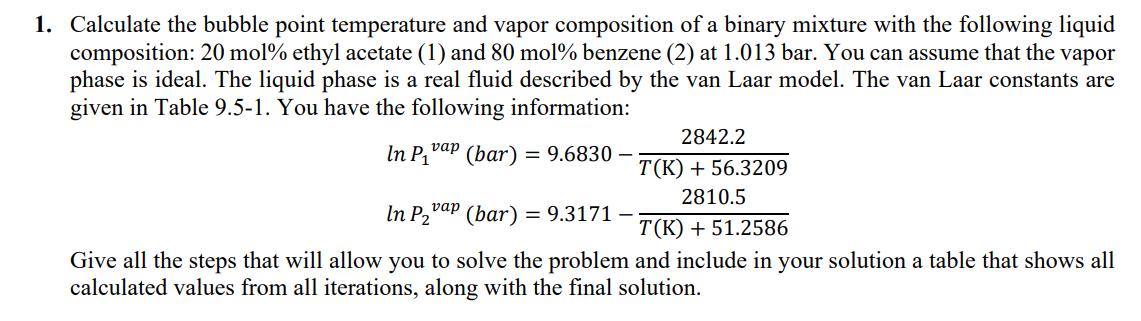
1. Calculate the bubble point temperature and vapor composition of a binary mixture with the following liquid composition: 20 mol% ethyl acetate (1) and 80 mol% benzene (2) at 1.013 bar. You can assume that the vapor phase is ideal. The liquid phase is a real fluid described by the van Laar model. The van Laar constants are given in Table 9.5-1. You have the following information: In P vap (bar) = 9.6830 In P vap (bar) = 9.3171 - 2842.2 T(K)+56.3209 2810.5 T(K) +51.2586 Give all the steps that will allow you to solve the problem and include in your solution a table that shows all calculated values from all iterations, along with the final solution.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Biochemical And Engineering Thermodynamics
Authors: Stanley I. Sandler
5th Edition
047050479X, 978-0470504796
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



