Question
2. The excess Gibbs energy for a binary mixture is given by Gex = x1x2(a + bT), where a and b are constants and
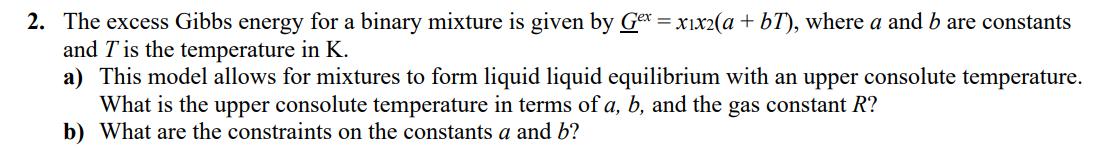
2. The excess Gibbs energy for a binary mixture is given by Gex = x1x2(a + bT), where a and b are constants and 7 is the temperature in K. a) This model allows for mixtures to form liquid liquid equilibrium with an upper consolute temperature. What is the upper consolute temperature in terms of a, b, and the gas constant R? b) What are the constraints on the constants a and b?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Engineering And Chemical Thermodynamics
Authors: Milo D. Koretsky
2nd Edition
0470259612, 978-0470259610
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



