Question
1). For which structure(s) does the electron-bearing carbon atom have a formal charge of zero? 2). For which structure(s) is the net charge zero? 0%
1). For which structure(s) does the electron-bearing carbon atom have a formal charge of zero?
2). For which structure(s) is the net charge zero?
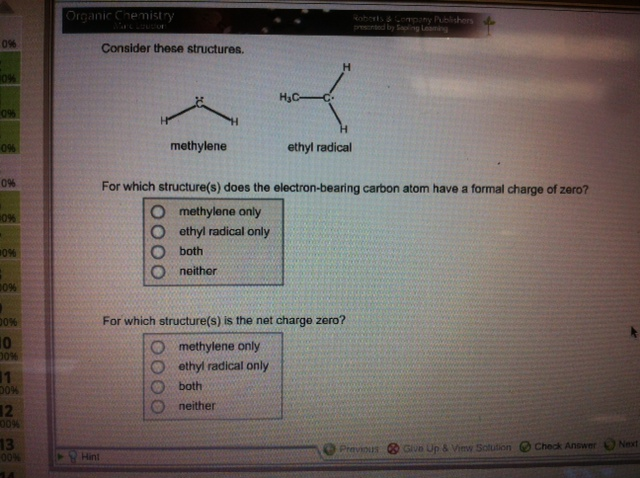
0% 10% 096 0% 0% 0% #0% 00% 00% 10 00% 1 30% 12 00% 13 00% 14 Organic Chemistry Hint Consider these structures. methylene H ethyl radical only O both Oneither HC- ethyl radical For which structure(s) does the electron-bearing carbon atom have a formal charge of zero? O methylene only O Roberts & Company Publishers presented by Sapling Leaming For which structure(s) is the net charge zero? methylene only ethyl radical only both neither Previous Give Up & View Solution Check Answer Next
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Answer and Explanation 1 1 Both 2 Both In both structures there carbon has a formal cha...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



