Answered step by step
Verified Expert Solution
Question
1 Approved Answer
0.0520 nm (a wavelength used in medical X rays) Express your answer using three significant figures. 195] xa Xbx Vxx E= 57.7 107 XX
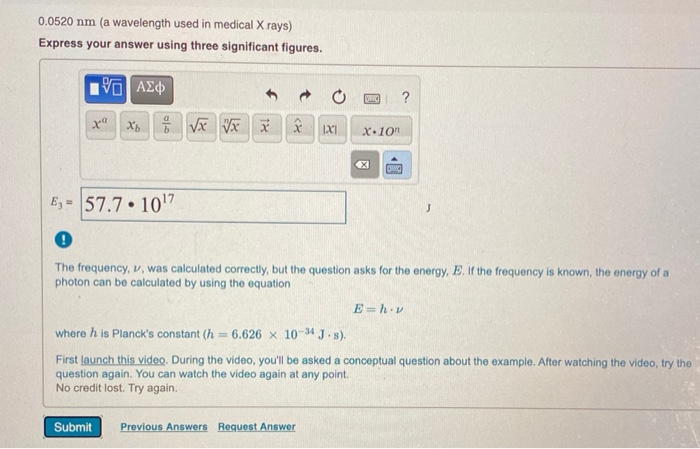
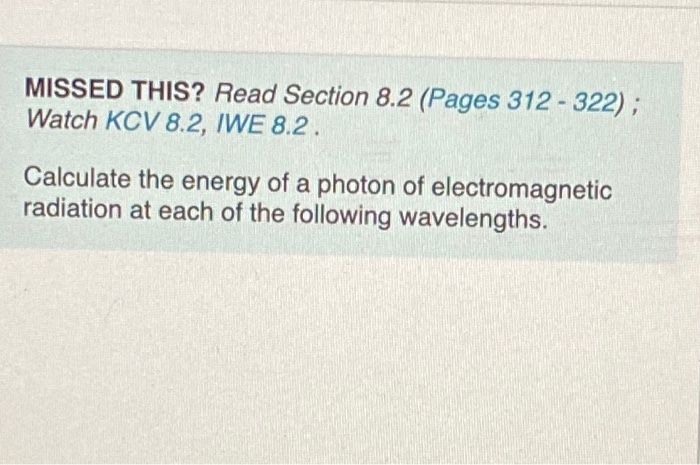
0.0520 nm (a wavelength used in medical X rays) Express your answer using three significant figures. 195] xa Xbx Vxx E= 57.7 107 XX Submit IXI D Previous Answers Request Answer X.10n ? ! The frequency, v, was calculated correctly, but the question asks for the energy, E. If the frequency is known, the energy of a photon can be calculated by using the equation OMC where h is Planck's constant (h= 6.626 x 10-34 J-s). First launch this video. During the video, you'll be asked a conceptual question about the example. After watching the video, try the question again. You can watch the video again at any point. No credit lost. Try again. E=h.v MISSED THIS? Read Section 8.2 (Pages 312-322); Watch KCV 8.2, IWE 8.2. Calculate the energy of a photon of electromagnetic radiation at each of the following wavelengths. 0.0520 nm (a wavelength used in medical X rays) Express your answer using three significant figures. 195] xa Xbx Vxx E= 57.7 107 XX Submit IXI D Previous Answers Request Answer X.10n ? ! The frequency, v, was calculated correctly, but the question asks for the energy, E. If the frequency is known, the energy of a photon can be calculated by using the equation OMC where h is Planck's constant (h= 6.626 x 10-34 J-s). First launch this video. During the video, you'll be asked a conceptual question about the example. After watching the video, try the question again. You can watch the video again at any point. No credit lost. Try again. E=h.v MISSED THIS? Read Section 8.2 (Pages 312-322); Watch KCV 8.2, IWE 8.2. Calculate the energy of a photon of electromagnetic radiation at each of the following wavelengths. 0.0520 nm (a wavelength used in medical X rays) Express your answer using three significant figures. 195] xa Xbx Vxx E= 57.7 107 XX Submit IXI D Previous Answers Request Answer X.10n ? ! The frequency, v, was calculated correctly, but the question asks for the energy, E. If the frequency is known, the energy of a photon can be calculated by using the equation OMC where h is Planck's constant (h= 6.626 x 10-34 J-s). First launch this video. During the video, you'll be asked a conceptual question about the example. After watching the video, try the question again. You can watch the video again at any point. No credit lost. Try again. E=h.v MISSED THIS? Read Section 8.2 (Pages 312-322); Watch KCV 8.2, IWE 8.2. Calculate the energy of a photon of electromagnetic radiation at each of the following wavelengths. 0.0520 nm (a wavelength used in medical X rays) Express your answer using three significant figures. 195] xa Xbx Vxx E= 57.7 107 XX Submit IXI D Previous Answers Request Answer X.10n ? ! The frequency, v, was calculated correctly, but the question asks for the energy, E. If the frequency is known, the energy of a photon can be calculated by using the equation OMC where h is Planck's constant (h= 6.626 x 10-34 J-s). First launch this video. During the video, you'll be asked a conceptual question about the example. After watching the video, try the question again. You can watch the video again at any point. No credit lost. Try again. E=h.v MISSED THIS? Read Section 8.2 (Pages 312-322); Watch KCV 8.2, IWE 8.2. Calculate the energy of a photon of electromagnetic radiation at each of the following wavelengths. 0.0520 nm (a wavelength used in medical X rays) Express your answer using three significant figures. 195] xa Xbx Vxx E= 57.7 107 XX Submit IXI D Previous Answers Request Answer X.10n ? ! The frequency, v, was calculated correctly, but the question asks for the energy, E. If the frequency is known, the energy of a photon can be calculated by using the equation OMC where h is Planck's constant (h= 6.626 x 10-34 J-s). First launch this video. During the video, you'll be asked a conceptual question about the example. After watching the video, try the question again. You can watch the video again at any point. No credit lost. Try again. E=h.v MISSED THIS? Read Section 8.2 (Pages 312-322); Watch KCV 8.2, IWE 8.2. Calculate the energy of a photon of electromagnetic radiation at each of the following wavelengths.
Step by Step Solution
★★★★★
3.46 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Eby 2 z he X 0052 nm ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


