Question
What is the reaction mechanism for this reaction? 1. H 11 H OH 2. ROH OH Camphor Borneol Isoborneol M.W. = 154.24 g/mole m.p. =
What is the reaction mechanism for this reaction? 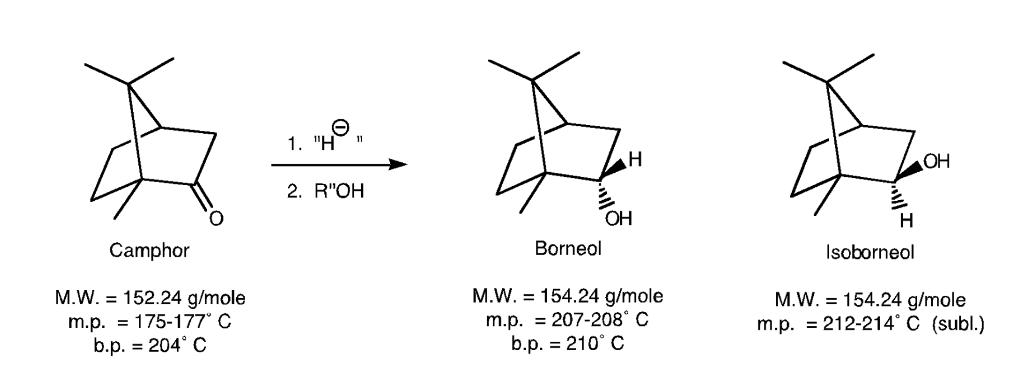
1. "H 11 H OH 2. R"OH OH Camphor Borneol Isoborneol M.W. = 154.24 g/mole m.p. = 207-208 C b.p. = 210 C M.W. = 152.24 g/mole M.W. = 154.24 g/mole m.p. = 212-214 C (subl.) m.p. = 175-177 C b.p. = 204 C 1. "H 11 H OH 2. R"OH OH Camphor Borneol Isoborneol M.W. = 154.24 g/mole m.p. = 207-208 C b.p. = 210 C M.W. = 152.24 g/mole M.W. = 154.24 g/mole m.p. = 212-214 C (subl.) m.p. = 175-177 C b.p. = 204 C 1. "H 11 H OH 2. R"OH OH Camphor Borneol Isoborneol M.W. = 154.24 g/mole m.p. = 207-208 C b.p. = 210 C M.W. = 152.24 g/mole M.W. = 154.24 g/mole m.p. = 212-214 C (subl.) m.p. = 175-177 C b.p. = 204 C
Step by Step Solution
3.41 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
OH poc...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial Accounting and Reporting
Authors: Barry Elliott, Jamie Elliott
14th Edition
978-0273744535, 273744445, 273744534, 978-0273744443
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



