Question
1) Half-life of an element is t second. Calculate the percentage left undecayed in t/2 sec. 2) 54.5 mg Na3PO4 contains 32^P (15.6%) and
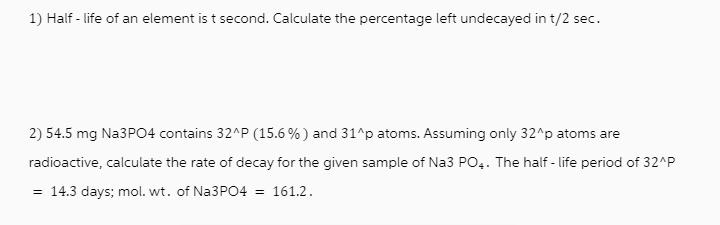
1) Half-life of an element is t second. Calculate the percentage left undecayed in t/2 sec. 2) 54.5 mg Na3PO4 contains 32^P (15.6%) and 31^p atoms. Assuming only 32^p atoms are radioactive, calculate the rate of decay for the given sample of Na3 PO4. The half-life period of 32^P = 14.3 days; mol. wt. of Na3PO4 = 161.2.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
1 Percentage left undecayed in t2 seconds The fraction of the element left undecayed after a specifi...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Modern Engineering Mathematics
Authors: Glyn James
6th Edition
1292253495, 9781292253497
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



