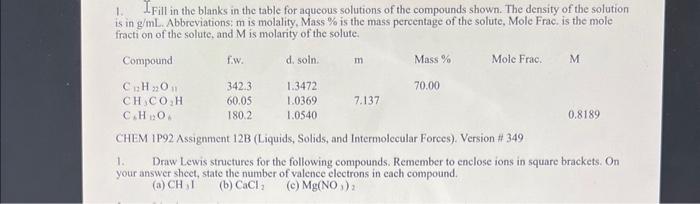
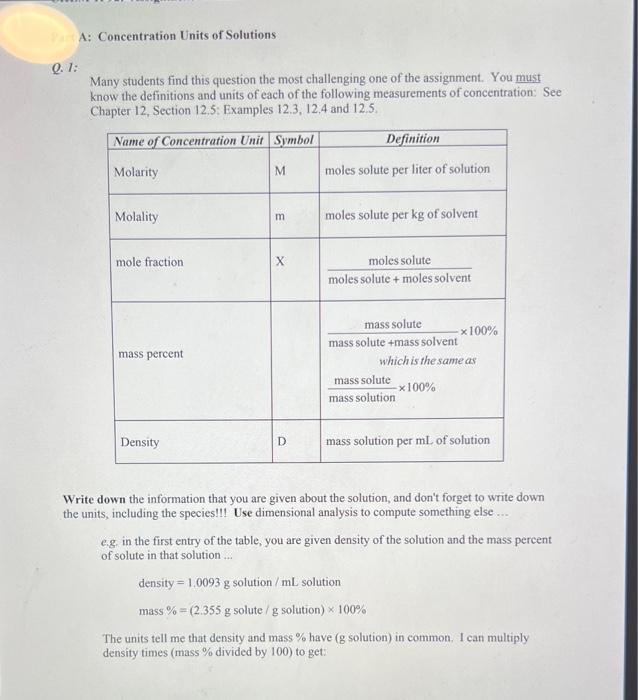
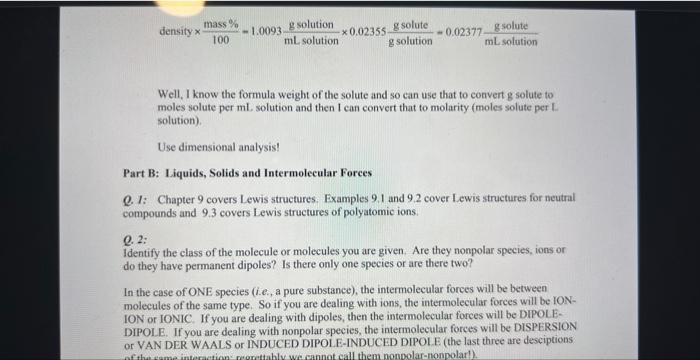
1. IFill in the blanks in the table for aqueous solutions of the compounds shown. The density of the solution is in g/mL. Abbreviations: m is molality, Mass \% is the mass percentage of the solute, Mole Frac, is the mole fracti on of the solute, and M is molarity of the solute. CHEM 1P92 Assignment 12B (Liquids, Solids, and Intermolccular Forces). Version \# 349 1. Draw Lewis structures for the following compounds. Remember to enclose ions in square brackets. On your answer sheet, state the number of valence electrons in each compound. (a) CH,I (b) CaCl2 (c) Mg(NO3)2 Q. I: Many students find this question the most challenging one of the assignment. You must know the definitions and units of each of the following measurements of concentration: See Chapter 12, Section 12.5: Examples 12.3, 12.4 and 12.5. Write down the information that you are given about the solution, and don't forget to write down the units, including the species!!! Use dimensional analysis to compute something else ... e.g. in the first entry of the table, you are given density of the solution and the mass percent of solute in that solution ... density =1.0093g solution /mL solution mass %=(2.355g solute /g solution )100% The units tell me that density and mass % have ( g solution) in common. I can multiply density times (mass % divided by 100 ) to get: density100mass%=1.0093mLsolutiongsolution0.02355gsolutiongsolute=0.02377mLsolutiongsolute Well, I know the formula weight of the solute and so can use that to convert g solute to moles solute per mL. solution and then I can convert that to molarity (moles solute per L. solution). Use dimensional analysis! Part B: Liquids, Solids and Intermolecular Forces Q. 1: Chapter 9 covers Lewis structures. Examples 9.1 and 9.2 cover Lewis structures for neutral compounds and 9.3 covers Lewis structures of polyatomic ions. Q. 2: Identify the class of the molecule or molecules you are given. Are they nonpolar species, ions or do they have permanent dipoles? Is there only one species or are there two? In the case of ONE species (i.e., a pure substance), the intermolecular forces will be between molecules of the same type. So if you are dealing with ions, the intermolecular forces will be IONION or IONIC. If you are dealing with dipoles, then the intermolecular forces will be DIPOLEDIPOLE. If you are dealing with nonpolar species, the intermolecular forces will be DISPERSION or VAN DER WAALS or INDUCED DIPOLE-INDUCED DIPOIE (the last three are desciptions