Question
1- In the following reaction: KMNO4 + NazCz04 + H2SO4 + CO2 + MnSO4 + NazSO4 + K2SO4 + H2O What is the mean oxidation
1-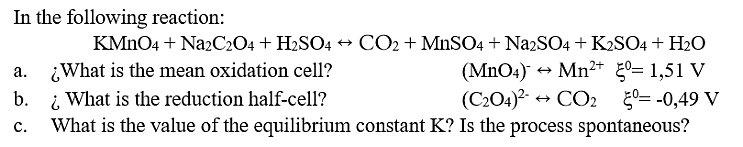
In the following reaction: KMNO4 + NazCz04 + H2SO4 + CO2 + MnSO4 + NazSO4 + K2SO4 + H2O What is the mean oxidation cell? i What is the reduction half-cell? What is the value of the equilibrium constant K? Is the process spontaneous? (MnO4) Mn+ 0= 1,51 V (C204)? + . b. + CO2 50= -0,49 V .
Step by Step Solution
3.47 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
If the standard electrode potential of the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Joseph M. Hornback
2nd Edition
9781133384847, 9780199270293, 534389511, 1133384846, 978-0534389512
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



