Question
1. Label the six phase changes of water on the diagram below (Figure 6.2). Solid Ice Liquid Water Figure 6.2: Phase Changes of Water.
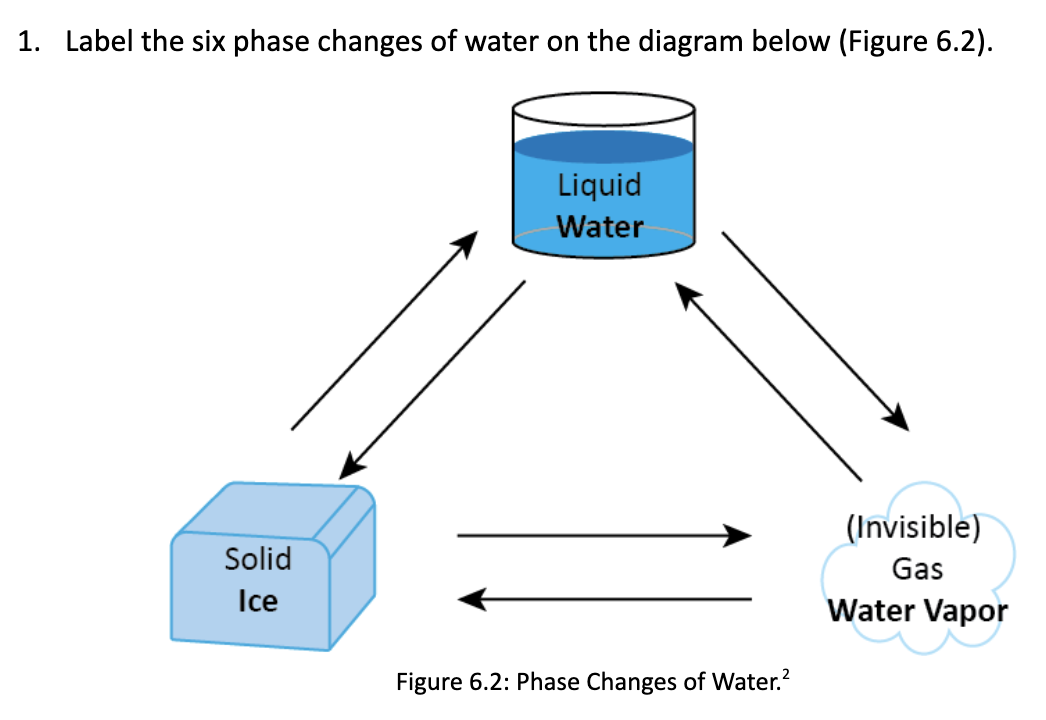
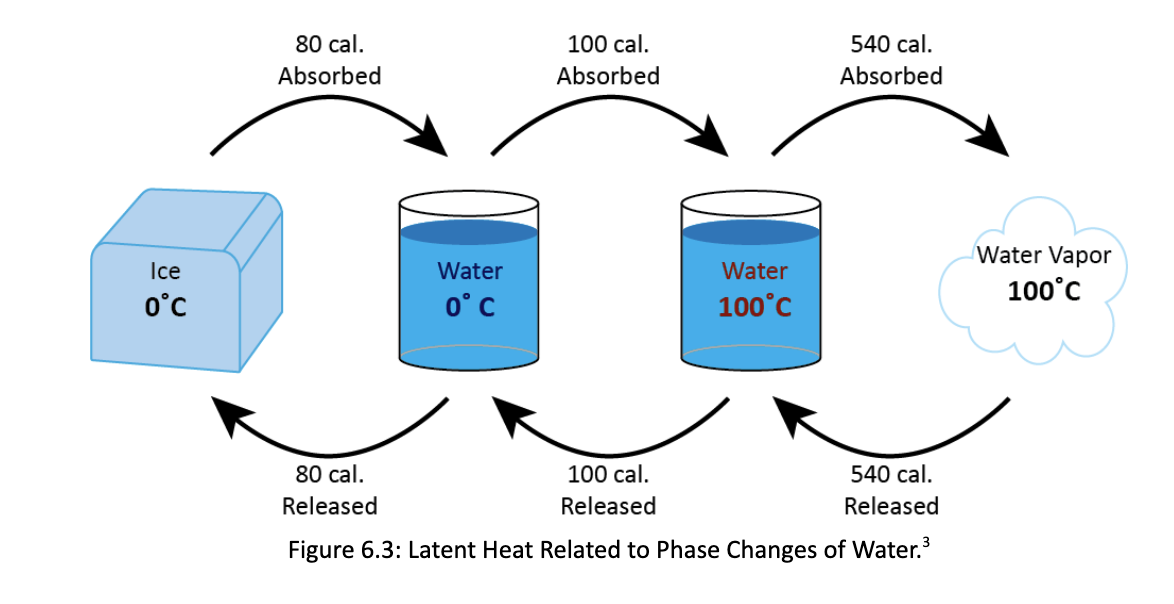
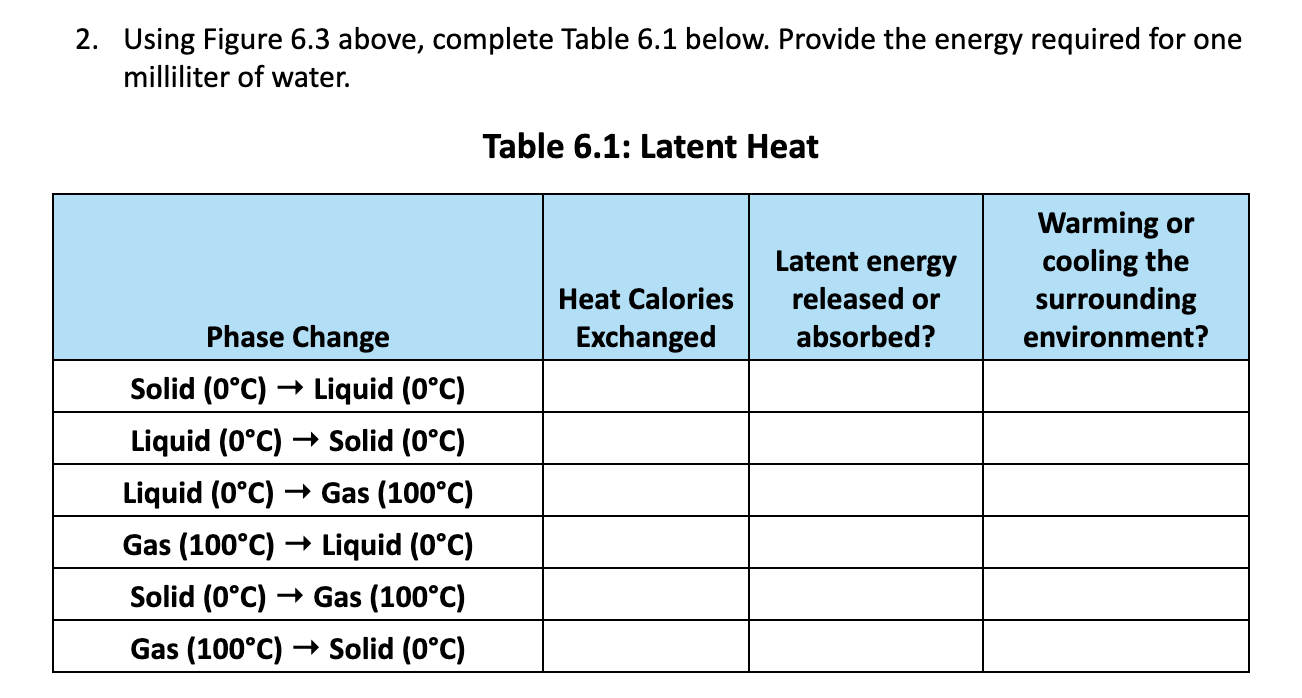
1. Label the six phase changes of water on the diagram below (Figure 6.2). Solid Ice Liquid Water Figure 6.2: Phase Changes of Water. (Invisible) Gas Water Vapor 80 cal. Absorbed 100 cal. Absorbed 540 cal. Absorbed Ice 0C Water Water 0C 100C 80 cal. Released 100 cal. Released 540 cal. Released Figure 6.3: Latent Heat Related to Phase Changes of Water. Water Vapor 100C 2. Using Figure 6.3 above, complete Table 6.1 below. Provide the energy required for one milliliter of water. Table 6.1: Latent Heat Phase Change Solid (0C) Liquid (0C) Liquid (0C) Solid (0C) Liquid (0C) Gas (100C) Gas (100C) > Liquid (0C) Solid (0C) Gas (100C) Gas (100C) Solid (0C) Warming or Latent energy cooling the Heat Calories released or surrounding Exchanged absorbed? environment?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles The Quest For Insight
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
7th Edition
1464183953, 9781464183959
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



