Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. Pig iron is obtained by charging ore, flax and coke into a blast furnace with the following compositions. The flux is in the ratio
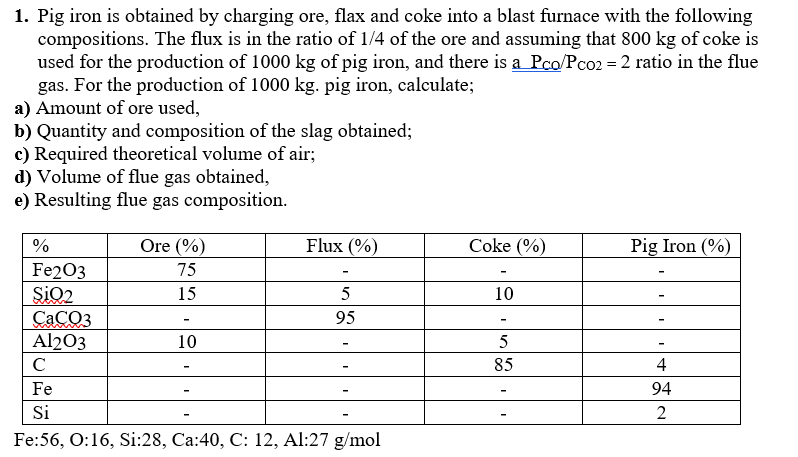 1. Pig iron is obtained by charging ore, flax and coke into a blast furnace with the following compositions. The flux is in the ratio of 1/4 of the ore and assuming that 800kg of coke is used for the production of 1000kg of pig iron, and there is aPCO/PCO2=2 ratio in the flue gas. For the production of 1000kg. pig iron, calculate; a) Amount of ore used b) Quantity and composition of the slag obtained; c) Required theoretical volume of air; d) Volume of flue gas obtained, e) Resulting flue gas composition. Fe: 56,O:16,Si:28,Ca:40,C:12,Al:27g/mol
1. Pig iron is obtained by charging ore, flax and coke into a blast furnace with the following compositions. The flux is in the ratio of 1/4 of the ore and assuming that 800kg of coke is used for the production of 1000kg of pig iron, and there is aPCO/PCO2=2 ratio in the flue gas. For the production of 1000kg. pig iron, calculate; a) Amount of ore used b) Quantity and composition of the slag obtained; c) Required theoretical volume of air; d) Volume of flue gas obtained, e) Resulting flue gas composition. Fe: 56,O:16,Si:28,Ca:40,C:12,Al:27g/mol Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


