Question
1. Sievert's law (8pt) At 440 degree C, under 10 atm of pure O2 gas, the dissolved oxygen (in atomic form) counts for 0.001
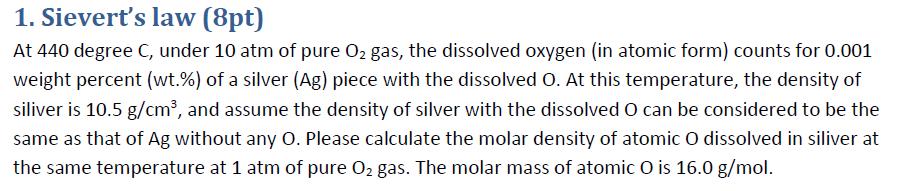
1. Sievert's law (8pt) At 440 degree C, under 10 atm of pure O2 gas, the dissolved oxygen (in atomic form) counts for 0.001 weight percent (wt.%) of a silver (Ag) piece with the dissolved O. At this temperature, the density of siliver is 10.5 g/cm, and assume the density of silver with the dissolved O can be considered to be the same as that of Ag without any O. Please calculate the molar density of atomic O dissolved in siliver at the same temperature at 1 atm of pure O2 gas. The molar mass of atomic O is 16.0 g/mol.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Sieverts Law and Molar Density of Dissolved Oxygen 1 We cannot directly apply Sieverts Law in this s...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals Of Momentum Heat And Mass Transfer
Authors: James Welty, Gregory L. Rorrer, David G. Foster
6th Edition
1118947460, 978-1118947463
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



