Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1 . The molar solubility of silver sulfite in a 0 . 1 2 2 M silver acetate solution is . . . M .
The molar solubility of silver sulfite in a silver acetate solution is
M
The maximum amount of cobaltII hydroxide that will dissolve in a cobaltII nitrate solution is M
S
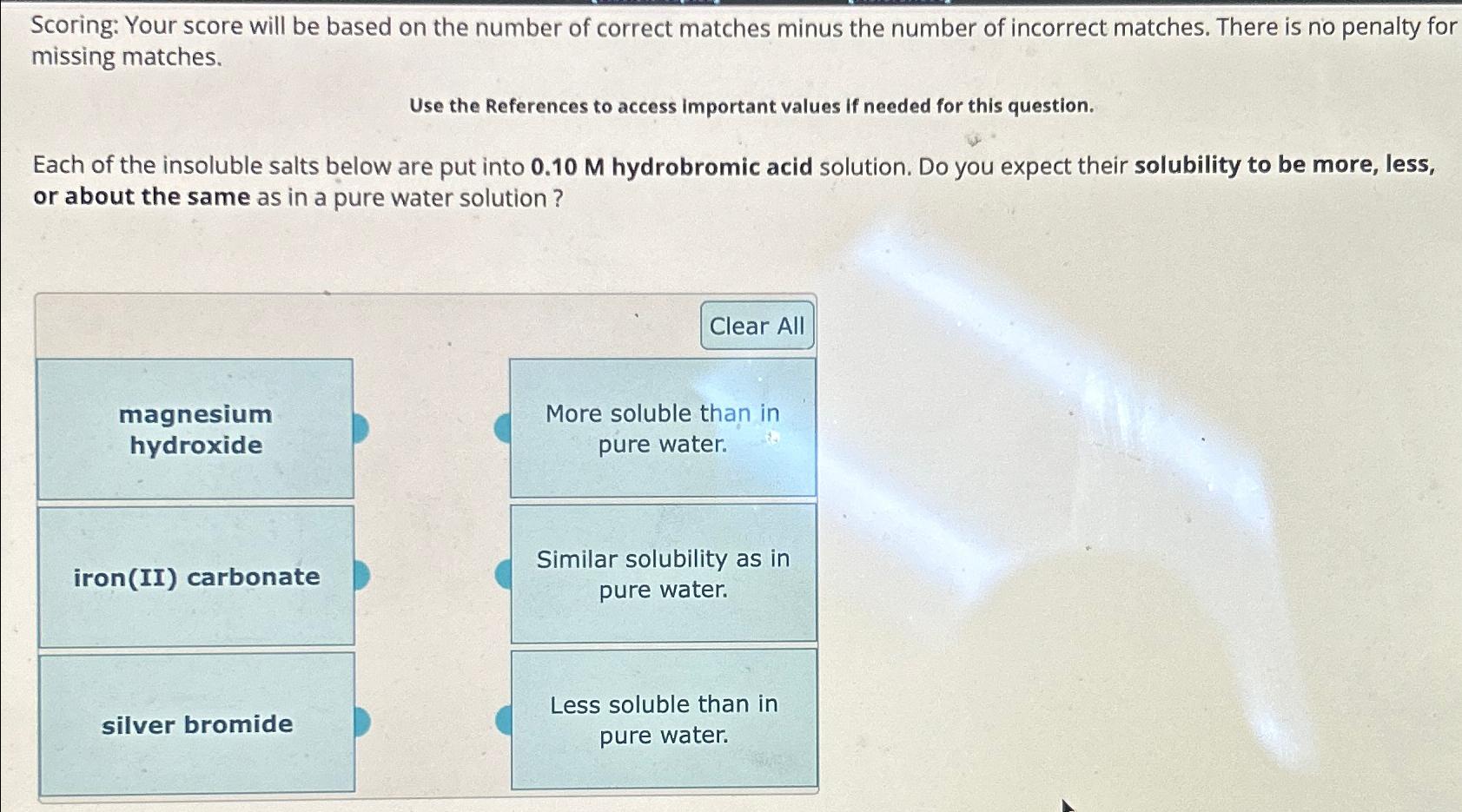
Scoring: Your score will be based on the number of correct matches minus the number of incorrect matches. There is no penalty for missing matches.
Use the References to access important values if needed for this question.
Each of the insoluble salts below are put into hydrobromic acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution?
chromiumIIIhydroxideMore soluble than in pure water.barium carbonateSimilar solubility as in pure waterlead bromideLess
Scoring: Your score will be based on the number of correct matches minus the number of incorrect matches. There is no penalty for missing matches.
Use the References to access important values if needed for this question.
Each of the insoluble salts below are put into hydrobromic acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution?
magnesium hydroxide
iron II carbonate
silver bromide
more soluble than in pure water.
Similar solubility as in pure water.
less soluble than in pure water.
Answer all pls I need help in all questions!!!!
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


