Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1) The vibrations of an Oxygen molecule, O2 are equivalent to those of harmonic oscillator with a force constant ky= 2294 N/m. Use m(160) =
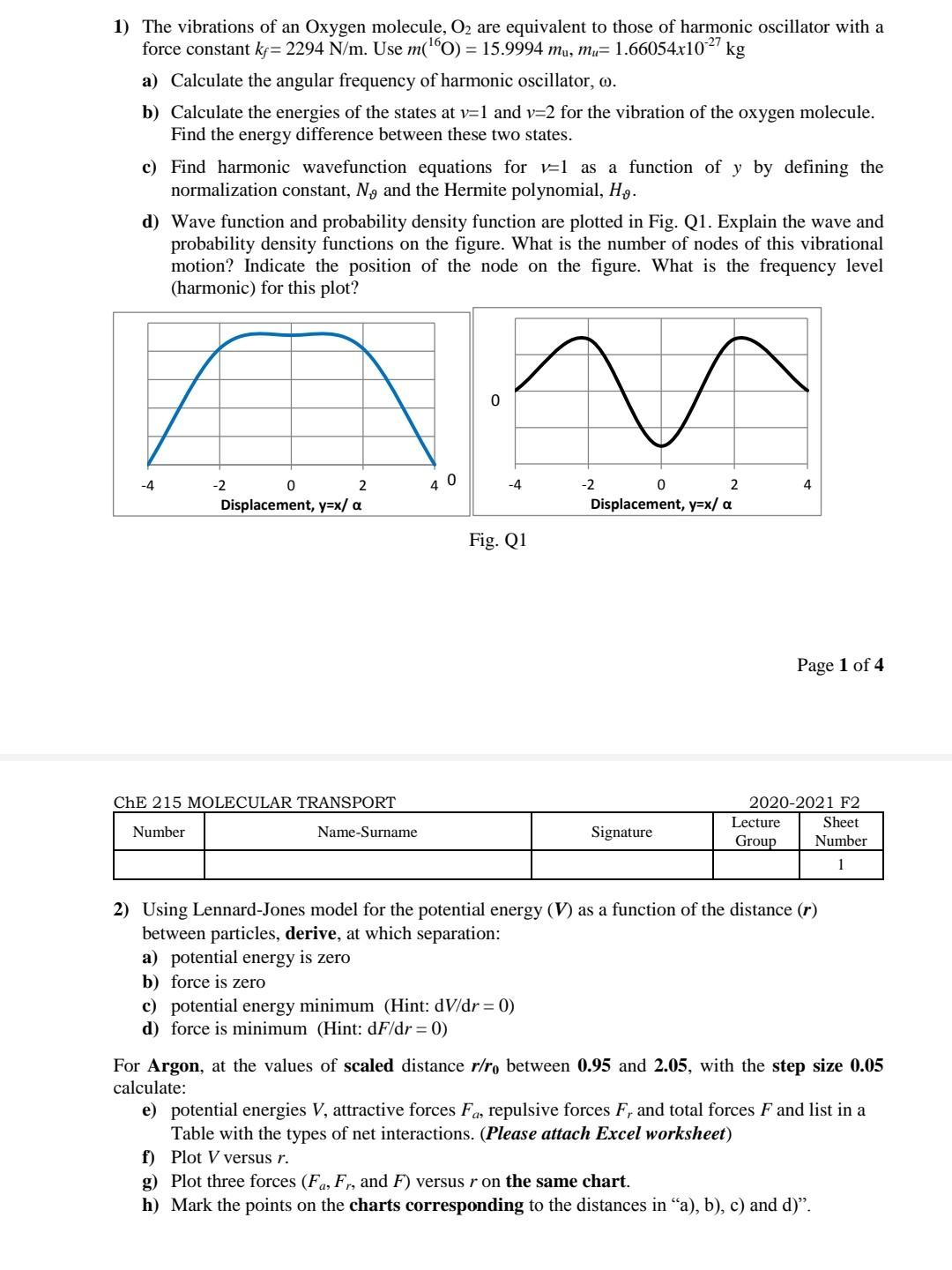
1) The vibrations of an Oxygen molecule, O2 are equivalent to those of harmonic oscillator with a force constant ky= 2294 N/m. Use m(160) = 15.9994 mu, mu= 1.66054x10-27 kg a) Calculate the angular frequency of harmonic oscillator, o. b) Calculate the energies of the states at v=1 and v=2 for the vibration of the oxygen molecule. Find the energy difference between these two states. c) Find harmonic wavefunction equations for v=l as a function of y by defining the normalization constant, N, and the Hermite polynomial, Hg. d) Wave function and probability density function are plotted in Fig. Q1. Explain the wave and probability density functions on the figure. What is the number of nodes of this vibrational motion? Indicate the position of the node on the figure. What is the frequency level (harmonic) for this plot? 0 -4 40 4 -2 0 2 Displacement, y=x/ a -2 2 Displacement, y=x/ a Fig. 21 Page 1 of 4 ChE 215 MOLECULAR TRANSPORT Number Name-Surname Signature 2020-2021 F2 Lecture Sheet Group Number 1 2) Using Lennard-Jones model for the potential energy (V) as a function of the distance (r) between particles, derive, at which separation: a) potential energy is zero b) force is zero c) potential energy minimum (Hint: dv/dr = 0) d) force is minimum (Hint: dF/dr=0) For Argon, at the values of scaled distance r/r, between 0.95 and 2.05, with the step size 0.05 calculate: e) potential energies V, attractive forces Fa, repulsive forces F, and total forces F and list in a Table with the types of net interactions. (Please attach Excel worksheet) f) Plot V versus r. g) Plot three forces (Fa, F., and F) versus r on the same chart. h) Mark the points on the charts corresponding to the distances in a), b), c) and d)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


