Question
1. To what temperature will 8200 JJ of heat raise 3.5 kg of water that is initially at 19.0 0 C? The specific heat of
1. To what temperature will 8200 JJ of heat raise 3.5 kg of water that is initially at 19.0 0C? The specific heat of water is 4186 J/kg?C0.
2. How much heat (in joules) is required to raise the temperature of 32.0 kg of water from 24 0C to 93 0C? The specific heat of water is 4186 J/kg?C0
3. (a). How much energy is required to bring a 1.6-L pot of water at 24 0C to 100 0C? The specific heat of water is 4186 J/kg?C0.
(b). For how long could this amount of energy run a 60-W lightbulb?
4. How much heat is needed to melt 12.50 kg of silver that is initially at 25 0C? The melting point of silver is 961 0C, the heat of fusion is 88 kJ/kg, the specific heat is 230 J/kg?C0.
5. High-altitude mountain climbers do not eat snow, but always melt it first with a stove. To see why, calculate the energy absorbed from a climber's body under the following conditions. The specific heat of ice is 2100 J/kg?C0, the latent heat of fusion is 333 kJ/kg the specific heat of water is 4186 J/kg?C0.
Calculate the energy absorbed from a climber's body if he eats 0.90 kg of -15 0C snow which his body warms to body temperature of 37 0C.
6. A cube of ice is taken from the freezer at -6.5 0C and placed in a 75-gg aluminum calorimeter filled with 300 g of water at room temperature of 20.0 0C. The final situation is observed to be all water at 17.0 0C. The specific heat of ice is 2100 J/kg?C0, the specific heat of aluminum is 900 J/kg?C0 , the specific heat of water is 4186 J/kg?C0, the heat of fusion of water is 333 kJ/Kg
What was the mass of the ice cube?
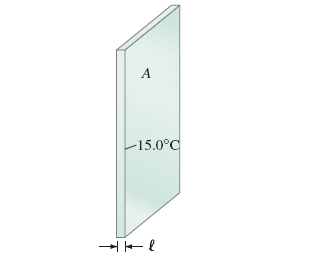
7. A major source of heat loss from a house in cold weather is through the windows.
Calculate the rate of heat flow by conduction through a glass window 2.0 m * 1.5 m in area and ? = 2.6 mm thick (Figure 1), if the temperature at the inner and outer surface is 15.0 0C. Assume that there are strong gusty winds and the external temperature is -12.0 0C.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


