Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. What is the shape of an organic molecule containing a triple bond with respect to the carbons on either side of the bond? Explain














1. What is the shape of an organic molecule containing a triple bond with respect to the carbons on either side of the bond? Explain your answer.
2. When looking at Newman projections are there instances where the staggered conformation is not the lowest in energy? Explain your answer.
3. Draw three different dibromocyclobutanes (there are six possibilities when including directionality).
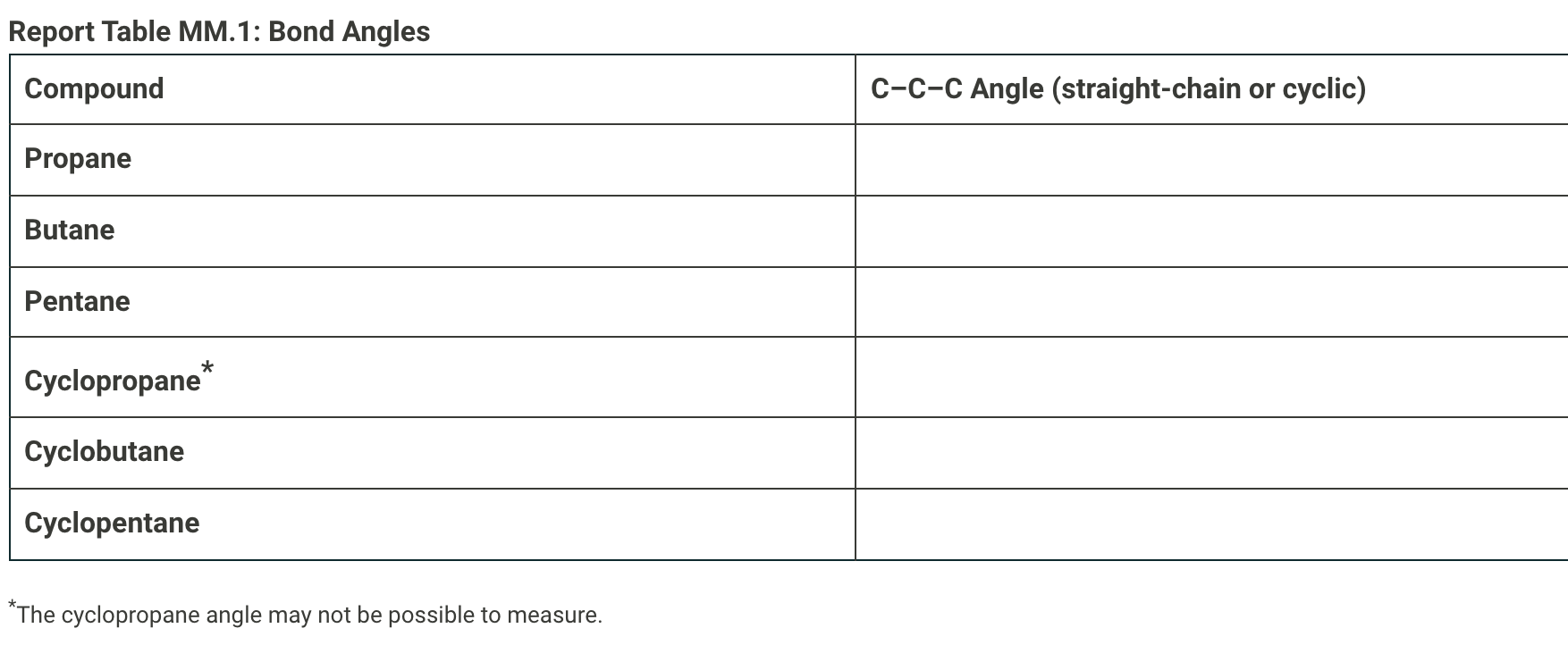
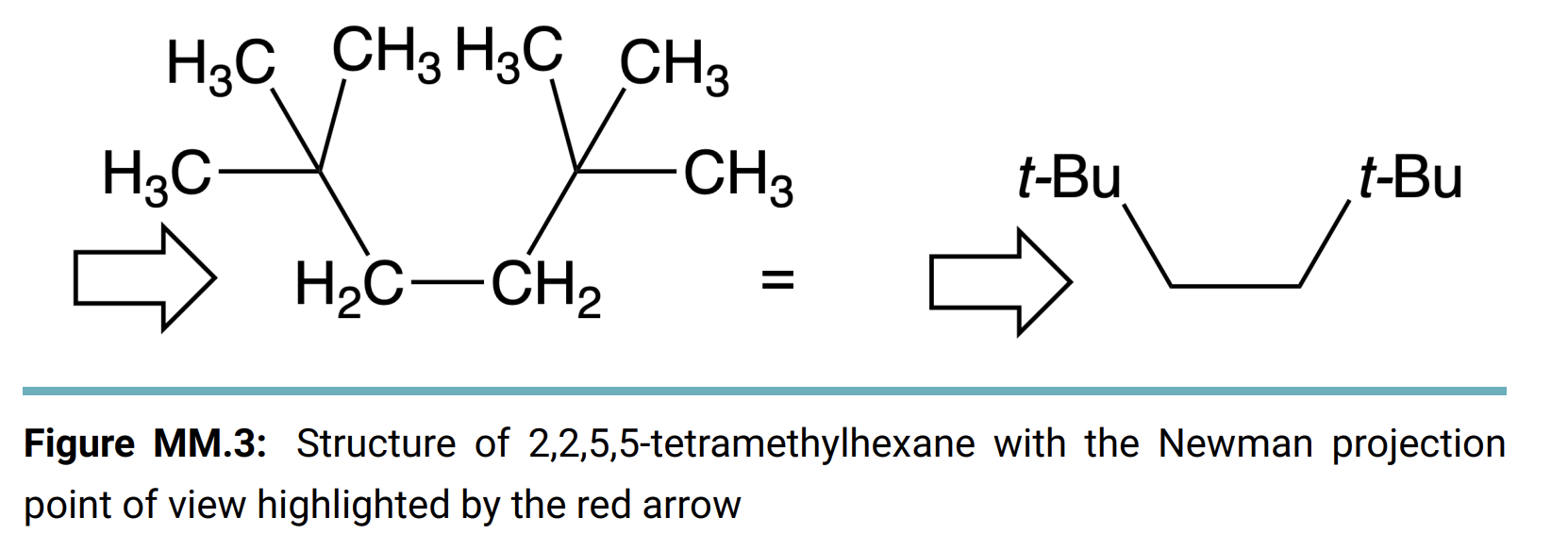
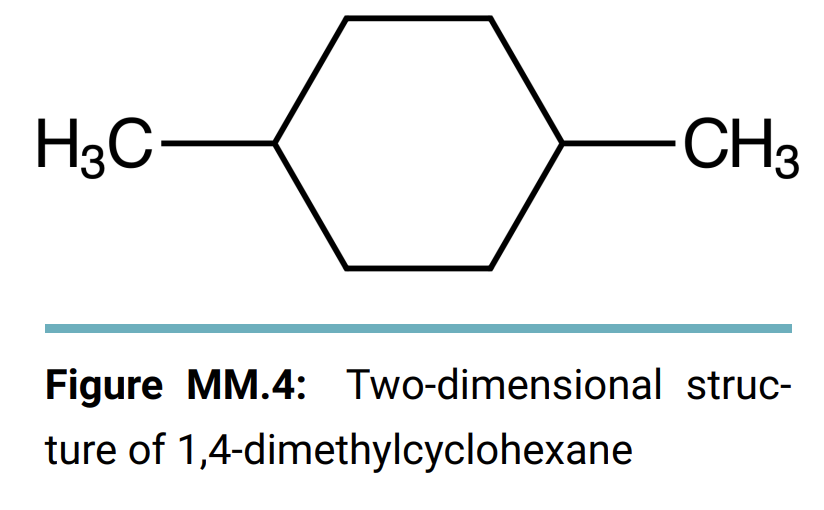
Molecular Modeling Kits Bond Angles Enter your data and observations into the textfields below. You can add images of structure drawings to the textfields by dragging images into the textfields. Alternatively, there is an image upload button in the tool bar of the text editor, you can click that button to open an upload menu to select your file to add an image. Once you add the image, you are able to resize, rotate and crop images that you add to your responses. From the procedure a 1. Make a model of propane and a separate model of cyclopropane. 2. Measure the bond angles between carbons 1 and 2 in the propane and the angle between any two carbons on the cyclopropane using a protractor. 3. Repeat for butane, cyclobutane, pentane, and cyclopentane. 4. Record your data on the data sheet. Complete the following table with the the C-C-C bonds angles for each compound. Report Table MM.1: Bond Angles Compound C-C-C Angle (straight-chain or cyclic) Propane Butane Pentane * Cyclopropane Cyclobutane Cyclopentane *The cyclopropane angle may not be possible to measure. Newman Projections From the procedure 1. Build a model of 2,2,5,5-tetramethylhexane. 2. Orient the model so that you are looking at the carbon with the arrow pointing to it in Figure 3. Align the bond to the next carbon in the chain so that it is directly behind the first carbon to match a Newman projection view. (See Figure MM.3 in the lab manual) 3. Spin the carbons on either side of the bond you're looking down to cycle through all three staggered and all three eclipsed positions of the substituents. 4. Draw all six positions as Newman projections on the data sheet and identify the position with the highest energy. H3C CH3 H3 CH3 HC CH3 t-Bu t-Bu H2C-CH2 II Figure MM.3: Structure of 2,2,5,5-tetramethylhexane with the Newman projection point of view highlighted by the red arrow Draw the six Newman projections of all of the different energy levels. Label each as staggered or eclipsed and rank in order from lowest energy to highest Cyclohexane Conformations From the procedure 1. Build a model of cyclohexane with two methyl groups at the one and four positions. 2. Adjust the model into the boat conformation with both methyl groups pointing up. (See Figure MM.4 in the Lab Manual) 3. Flip one side of the ring down to transform the boat conformation into the chair conformation. 4. Answer the questions on the data sheet about the cis and trans arrangements in the boat and chair conformations. H3C CH3 Figure MM.4: Two-dimensional struc- ture of 1,4-dimethylcyclohexane Draw the four possible positions, the cis and trans positions of the methyl groups for both the chair and boat conformations, of 1,4- dimethylcyclohexane. Label each as cis or trans and identify the highest in energy. Benzene From the procedure 1. Build a model of benzene (1,3,5-cyclohexatriene). 2. Take note of the overall shape of the benzene molecule. 3. Answer the question on the data sheet. Referring to your model, explain why there is no directionality for a substituent group bonded directly to a benzene ring. Post Lab Questions
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


